
External beam radiation treatment of intrahepatic cholangiocarcinoma: a narrative review
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer after hepatocellular carcinoma (HCC) (1). It is an aggressive tumor with a high mortality due to its refractory nature and often presenting as advanced disease. Most patients die within a year of diagnosis due to local disease progression, resulting in biliary obstruction leading to liver failure or biliary sepsis (2).
An effective multidisciplinary approach is the optimal strategy for achieving the best outcomes in patients with ICC. In surgical candidates, curative intent resection is the preferred treatment option. However, up to 70% of patients have locoregional recurrence after surgery, suggesting a role for adjuvant radiation, although this is not clearly defined (3). In non-surgical candidates, systemic therapy followed by locoregional treatments such as definitive radiation may be considered. However, there is a wide variety in the types of radiation that can be offered, including conventional, ablative hypofractionated, and stereotactic body radiation therapy (SBRT), and it may be unclear as to when to offer each type. This review paper will focus on summarizing the role of radiation in the adjuvant and definitive setting for ICC. Outcomes of interest include overall survival (OS) and local control (LC). We present the following article in accordance with the Narrative Review checklist (available at http://dx.doi.org/10.21037/dmr-20-158).
Methods
We performed a systemic search of MEDLINE/PubMed and ClinicalTrials.gov databases, focusing on those published in the last 20 years. Our search queried “intrahepatic cholangiocarcinoma radiotherapy” and was limited only to clinical trials, meta-analyses, and retrospective studies, omitting books, documents, and reviews. Our search resulted in 55 references. These were manually reviewed, and only 20 references were within our scope of interest ranging from 2002–2018. There were 2 ongoing clinical trials, 1 phase III trial, 5 phase II trials, and 12 retrospective studies included in our review. Patients mostly had ICC, although some studies included patients with extrahepatic cholangiocarcinoma (ECC), gallbladder cancer, and HCC. The narrative review draws most of its interpretations from studies focusing solely on ICC, with priority on prospective trials followed by retrospective studies. For some studies with mixed populations, the results were interpreted by focusing on the ICC cohort if it was reported. If the results from the mixed populations were not separated, we would interpret its findings in the context of other published reports on related subjects.
Discussion
Adjuvant radiation for resected ICC
Curative treatment for ICC is complete resection of the affected segments or lobes of the liver. There are several studies examining factors affecting survival after resection of ICC. A retrospective review of 70 cases identified residual tumor status and pathological differentiation as independent factors predicting survival (4). Another retrospective review of 224 cases found that patients with hepatolithiasis, periductal infiltrative or periductal infiltrative mixed with mass-forming growth, higher T stage, and more advanced stage tended to have higher positive resection margin rates after hepatectomy (5). The liver parenchyma was the most common site of positive margin, followed by the bile duct and soft tissues. Locoregional recurrence was the most common pattern of recurrence, implying a role for radiation to improve local control.
A Surveillance, Epidemiology, and End Results (SEER) analysis found improved survival in ICC patients receiving surgery followed by radiation (6). The study had a total of 3,839 patients divided into four groups, one group receiving surgery with adjuvant radiation (N=286), one receiving surgery alone (N=948), one receiving definitive radiation (N=396), and one receiving no treatment (N=2,209). Patients receiving surgery with adjuvant radiation had the highest median survival at 11 months, compared to 6 months for surgery alone, 7 months for definitive radiation, and 3 months for patients not receiving any treatment. This study was limited in that it was not able to assess the margin status or lymph node status. Despite this, the study provides strong evidence that in ICC patients who are eligible for surgery, surgery followed by adjuvant radiation has the best outcomes.
Another study found a survival benefit with adjuvant radiation in ICC patients with concurrent regional lymph node metastases (7). This was a retrospective study of 90 patients with positive lymph nodes, 24 of whom received adjuvant radiation and 66 of whom underwent observation. Patients receiving adjuvant radiation received a median total dose of 50 Gy (range, 34–60 Gy) in 2 Gy fractions. It was found that patients receiving adjuvant radiation had a higher OS of 19.1 months compared to 9.5 months in patients undergoing observation. Multivariate analysis showed that increasing age, multiple intrahepatic primary tumors, higher level of CA 19-9, and non-radiotherapy group were related to a poorer prognosis. The most common cause of death was intrahepatic recurrence. This study suggests that in patients with resected ICC and positive lymph nodes, adjuvant radiation should be strongly considered.
There was one controversial study that suggested adjuvant radiation does not improve survival in ICC patients with margin-positive, node-negative disease. This was a National Cancer Database (NCDB) review of 2,897 patients with early stage (T1-3) ICC (8). Survival outcomes were examined following propensity score matching, and a Cox regression for survival analysis was used to examine predictors of survival. Radiation was delivered to 525 patients (255 with an R0 resection, 230 with an R1 or R2 resection, and 43 unknown). Radiation was associated with a trend toward an improved survival among patients with R1/R2 resection and lymph node negative patients (39.5 vs. 21.1 months). However, in a propensity matched cohort and by Cox regression analysis, radiation was not associated with survival, which led authors to conclude that radiation does not provide a survival benefit. This study was criticized for lacking information about the dose and timing of radiation, whether it was given adjuvant for local control or later for palliation. Results should be interpreted with caution given the presence of conflicting data.
Another NCDB review showed a survival benefit with adjuvant treatment in ICC patients with certain high risk features (9). This study identified 2,813 patients, of whom 42.3% received adjuvant treatment. It was found that adjuvant treatment after resection was associated with improved survival in patients with positive margins, positive nodes, and stage III/IVA disease. However, this study failed to differentiate what type of adjuvant treatment patients received, whether it be radiation, chemotherapy, or chemoradiation. This raises the question of which form of adjuvant treatment is superior.
Currently, there is no randomized data comparing the different adjuvant treatments for ICC. There is a phase II study (SWOG S0809) using chemotherapy followed by chemoradiation in resected ECC and gallbladder carcinoma worthy of mention (10). This study included 79 patients with ECC (68%) or gallbladder carcinoma (32%) after radical resection (R0, N=54; R1, N=25), stage pT2-4 or N+ or positive resection margins, M0, and performance status 0 to 1. Patients received adjuvant gemcitabine and capecitabine for 4 cycles followed by chemoradiation using capecitabine. With a 3D-conformal radiotherapy (3D-CRT) technique, the radiation dose to the regional lymphatics was 45 Gy, and the radiation dose to the tumor bed was 54 Gy for R0 resection and 59.4 Gy for R1 resection. With an intensity-modulated radiotherapy (IMRT) technique, radiation dose to the regional lymphatics was 45 Gy in 25 fractions with a simultaneous integrated boost (SIB) to the tumor bed of 52.5 Gy for R0 resection and 55 Gy for R1 resection. Patients had excellent outcomes with 2-year survival rate at 65% (67% for R0, 60% for R1). Median OS was 35 months (34 months for R0, 35 months for R1). Local, distant, and combined relapse occurred in 14, 24, and 9 patients respectively. Grade 3 and 4 adverse effects were 52% and 11% respectively. The most common grade 3 to 4 adverse effects were neutropenia (44%), hand-foot syndrome (11%), diarrhea (8%), lymphopenia (8%), and leukopenia (6%). There was one death resulting from gastrointestinal (GI) hemorrhage. This combination was well tolerated, has promising efficacy, and provides clinicians with a well-supported regimen. Future phase III trials are currently being planned.
Currently, the only phase III study examining adjuvant systemic therapy after resection is the BILCAP trial (11). Four hundred forty seven patients with biliary tract cancer (84 with ICC) after R0 or R1 resection were randomized to receive capecitabine or undergo observation. Patients receiving capecitabine had a trend towards improved OS (51.1 vs. 36.4 months) in the intention-to-treat analysis and a significantly improved OS (53 vs. 36 months) in the prespecified per-protocol analysis. Adjuvant capecitabine is now standard of care for resected R0/R1 ICC.
There is an ongoing phase III study that aims to compare adjuvant chemoradiation versus adjuvant chemotherapy after curative-intent resection: the ACTICCA-1 trial (12). This study has an estimated enrollment of 781 patients with cholangiocarcinoma including ICC and muscle invasive gallbladder carcinoma after R0 or R1 resection. Broadly, patients are randomized to receive gemcitabine and cisplatin (experimental) versus capecitabine (active comparator). In the experimental arm, there is an embedded sub-study randomizing R1 resected patients to chemoradiation (6 cycles of gemcitabine and cisplatin followed by IMRT with capecitabine) versus chemotherapy alone (8 cycles of gemcitabine and cisplatin). In the active comparator arm, the embedded sub-study randomizes R1 resected patients to chemoradiation (6 cycles of capecitabine followed by IMRT with capecitabine) versus chemotherapy alone (8 cycles of capecitabine). The primary endpoint is disease free survival (DFS), and secondary endpoints include recurrence free survival (RFS), OS, safety and tolerability of adjuvant chemoradiation, quality of life, and patterns of disease recurrence.
In summary, there is a lack of level 1 evidence for adjuvant radiation for ICC after surgical resection. The American Society of Clinical Oncology (ASCO) guidelines recommend all patients to receive adjuvant capecitabine per the BILCAP trial but do not suggest a role for adjuvant radiation for ICC (13). The European Society of Medical Oncology (ESMO) guidelines are less specific and suggest a multidisciplinary team approach for ICC considering adjuvant radiation, chemotherapy, or chemoradiation (14). The National Comprehensive Cancer Network (NCCN) guidelines provide the most comprehensive recommendations and are based on margin status and nodal status (15). For R0 resection, patients may undergo observation, adjuvant chemotherapy, or a clinical trial. For R1 resection or positive nodes, patients may undergo adjuvant chemotherapy, chemotherapy followed by chemoradiation, chemoradiation followed by chemotherapy, or a clinical trial. For R2 resection, patients should be treated as if they have unresectable disease, which will be covered in the next section. Ultimately, adjuvant radiation for ICC is still investigational and may be considered for margin-positive or node-positive disease. Radiation treatment should cover the draining regional lymph nodes to 45 Gy, and tumor bed (especially the area with positive margin) to 54–59.4 Gy in 1.8 Gy per fraction. Table 1 summarizes the presented data in this section.
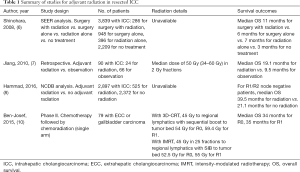
Full table
Definitive radiation for unresectable locally advanced ICC
For patients with unresectable locally advanced ICC, the median survival remains dismal, between 2.3 to 12 months (16). Reasons for unresectability includes multiple intrahepatic tumors, locally advanced disease in the liver with major vascular invasion, and nodal or distal metastases (17). Based on the results of the ABC-02 trial, doublet gemcitabine and cisplatin therapy is currently considered the standard-of-care first-line therapy for patients with advanced disease (16). Because the majority of patients eventually fail at the primary tumor site (88.3% by the ABC-02 trial) (16), there has been increasing interest in locoregional therapy following doublet chemotherapy. Patients have a variety of options for locoregional therapy, including transarterial chemoembolization (TACE), transarterial radioembolization (TARE), radiofrequency ablation (RFA), hepatic artery pump therapy, and external beam radiotherapy, including SBRT (18). For the purposes of this review, we will be only focused on external beam radiotherapy.
A retrospective study examined the role of external beam radiotherapy in unresectable ICC (19). The study included 84 patients, 35 of whom received radiation and 49 of whom did not. The median dose for patients receiving radiation was 50 Gy (ranging from 30–60 Gy). Patients who received radiation had improved OS compared to patients who did not (9.5 vs. 5.1 months). The compete and partial response rates in patients receiving radiation was 8.6% and 28.5% respectively. In 19 patients with jaundice who received radiation, complete and partial relief was observed in 26.8% and 31.6% respectively. This study showed that external beam radiotherapy improved prognosis and relieved jaundice symptoms in patients with unresectable ICC. However, it did not address definitive or ablative doses of radiation in the treatment of unresectable ICC.
A phase I study of SBRT for HCC and ICC was performed to determine the safety and efficacy of SBRT (20). 41 patients with unresectable HCC (N=31) or ICC (N=10) were included in this study, with a median dose of 36 Gy (ranging from 24–54 Gy) in 6 fractions. There was no radiation-induced liver disease or treatment-related grade 4/5 toxicities within the first 3 months after SBRT. Median OS of HCC and ICC patients was 11.7 and 15.0 months respectively. Authors concluded that six-fraction SBRT is a safe and effective treatment for unresectable HCC and ICC.
A dose-escalation study of single-fraction SBRT for liver malignancies attempted to determine the maximum safe dose of SBRT (21). This study included 26 patients with 40 identifiable lesions (HCC, N=2; ICC, N=5; hepatic metastases, N=19). The radiation dose was escalated from 18 Gy at 4 Gy increments with a planned maximum dose of 30 Gy. The radiation dose was safely escalated to the planned maximum dose of 30 Gy with 9 acute grade 1 toxicities, 1 acute grade 2 toxicity, and 2 late grade 2 toxicities. The cumulative risk of liver failure at 12 months was 23%. This study concluded that it is feasible and safe to deliver single-fraction, high-dose SBRT up to 30 Gy to a primary or metastatic liver lesion.
Several trials have since examined OS and LC outcomes following SBRT for ICC. One retrospective review of 58 cholangiocarcinoma patients treated with a median dose of 45 Gy in 3 fractions showed a median OS of 10 months, a 1-year LC rate of 85%, and a 2-year LC rate of 72% (22). Grade 3–4 toxicities (9%) included duodenal/gastric ulcers, cholangitis, gastric perforation, or bile duct stenosis. Another retrospective review of 34 patients with 42 lesions (ICC, N=31, perihilar cholangiocarcinoma, N=11) treated with a median dose of 30 Gy in 3 fractions showed a median OS of 17 months, median PFS of 10 months, a 1-year LC rate of 88%, and a 4-year LC rate of 79% (23). Grade 3 toxicities (12%) included duodenal ulcers, cholangitis, and liver abscess. Another retrospective review of 31 patients (ECC, N=25; ICC, N=6) treated with a median dose of 40 Gy in 5 fractions showed a median OS of 15.7 months, median time to progression 16.8 months, 1-year LC rate of 78%, and a 2-year LC rate of 47% (24). Specifically, all 9 local recurrence were in the high-dose SBRT fields. Grade 3 toxicities (16%) were not specified, but all patients who experienced severe late toxicity were prescribed a dose of 40 Gy in 5 fractions or higher. Another retrospective review of 37 patients with 43 lesions (ICC, N=17; ECC, N=26) were treated with SBRT using 3 ablative regimens depending on the proximity to organs at risk (OAR). 12.5 Gy ×3 fractions were given to lesions close to OARs, 4–5.5 Gy ×12 fractions were delivered to lesions with direct contact to OARs, and 7–10 Gy ×5 fractions were preferred in all other cases. A median dose of 45 Gy in 3–12 fractions were delivered. The study showed a median OS of 14 months from start of SBRT and 22 months from diagnosis and median PFS of 9 months (25). Grade 3 bleeding occurred in 9% of patients and grade 3 cholangitis occurred in 19% of patients. To summarize, ICC patients treated with SBRT had favorable outcomes, with median OS ranging from 10–22 months, 1-year LC rates ranging from 78–88%, and grade 3 toxicities ranging from 9–19%. An example of a SBRT treatment plan can be seen in Figure 1.
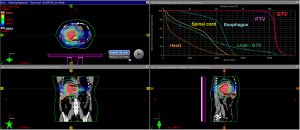
To reduce GI and biliary toxicities, for patients with large ICC, centrally located tumor (within 2 cm of the porta hepatis), or disease abutting critical structures, it is believed that conventional or ablative hypofractionated external beam radiation is the preferred locoregional treatment over SBRT. A single-arm, phase II, multi-institutional study examined high-dose hypofractionated proton beam radiotherapy in the treatment of unresectable HCC and ICC (26). The study included 44 patients with HCC and 37 patients with ICC. The median dose delivered to both HCC and ICC was 58 Gy in 15 fractions. The 2-year LC rate was 94.8% and 94.1% for HCC and ICC respectively, and the 2-year OS rate was 63.2% and 46.5% respectively. These findings demonstrate that high dose hypofractionated proton therapy has excellent LC, supporting ongoing phase III trials.
A retrospective dose response analysis attempted to determine the optimal ablative biological equivalent dose (BED) cutoff for improved outcomes (27). This study included 79 patients with ICC treated with 35–100 Gy in 3–30 fractions using an SIB technique with a median biological equivalent dose (BED) of 80.5 Gy (range, 43.75–180 Gy). It was found that radiation dose was the single most important prognostic factor, with higher doses correlating with improved OS and local control (LC). Patients treated with a BED >80.5 Gy had improved OS and LC compared to patients treated with a BED ≤80.5 Gy (3-year OS rate was 73% vs. 38%, 3-year LC was 78% vs. 45% respectively). Treatment was generally well-tolerated, with only 9% of patients developing biliary stenosis. This study suggests that any definitive radiation dose for ICC should aim to exceed a BED of 80.5 Gy.
To evaluate whether the addition of radiotherapy to chemotherapy affects survival, NRG-GI001 randomized patients with inoperable localized ICC to receive ablative radiation therapy versus observation following gemcitabine and cisplatin chemotherapy. The prescription radiation dose was 67.5, 45, or 37.5 Gy in 15 fractions based on normal tissue constraints. This highly anticipated clinical trial, however, has been terminated early due to poor accrual (28).
In summary, for patients with locally advanced unresectable ICC, standard treatment includes doublet chemotherapy using gemcitabine and cisplatin. If imaging studies show no evidence of systemic disease by the end of chemotherapy, locoregional treatment should be followed. For patients with multifocal or diffuse ICC, arterially directed therapies such as TACE or TARE are preferred. Otherwise, external beam radiation treatment should be considered. For small peripherally located lesions, SBRT up to 30–50 Gy in 3–5 fractions is recommended. For large ICC, centrally located tumors (within 2 cm of the porta hepatis), or lesions abutting critical structures, conventional external beam radiation up to a total dose of 59.4 Gy in 33 fractions by 3D-CRT or 55 Gy in 25 fractions by IMRT using SIB technique is recommended (15). Ablative hypofractionation, 67.5 Gy in 15 fractions or 75 Gy in 25 fractions, are considered investigational and should only be done at centers with experience (27). Table 2 summarizes the presented data in this section.
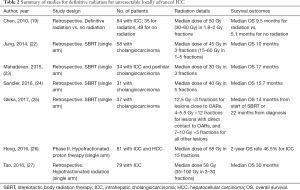
Full table
Summary
ICC is an aggressive disease with a high chance of locoregional recurrence. Radiation plays an important role in both the adjuvant and definitive setting given its ability to improve locoregional control. Curative treatment for operable ICC is surgical resection. In the presence of positive margins or positive nodes, adjuvant treatment in the form of radiation, chemotherapy, or chemoradiation is warranted. In locally advanced disease, doublet chemotherapy with gemcitabine and cisplatin is the mainstay of treatment. Radiation may be offered afterwards in the form of SBRT for small peripherally located lesions or in the form of conventional or hypofractionated regimens in large tumors abutting critical structures. As always, an effective multidisciplinary approach offers patients with ICC the best chance of long-term survival.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Digestive Medicine Research for the series “Locoregional and systemic treatment in intrahepatic cholangiocarcinoma”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the narrative review checklist. Available at http://dx.doi.org/10.21037/dmr-20-158
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-20-158). The series “Locoregional and systemic treatment in intrahepatic cholangiocarcinoma” was commissioned by the editorial office without any funding or sponsorship. YJC served as the unpaid Guest Editor of the series and serves as the unpaid editorial board member of Digestive Medicine Research from Mar 2020 to Feb 2022. The authors have no other conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004;24:115-25. [Crossref] [PubMed]
- Patel T. Cholangiocarcinoma-controversies and challenges. Nat Rev Gastroenterol Hepatol 2011;8:189-200. [Crossref] [PubMed]
- Song S, Kim K, Chie EK, et al. Locoregional recurrence after curative intent resection for intrahepatic cholangiocarcinoma: implications for adjuvant radiotherapy. Clin Transl Oncol 2015;17:825-9. [Crossref] [PubMed]
- Murakami Y, Uemura K, Sudo T, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol 2011;18:651-8. [Crossref] [PubMed]
- Yeh CN, Hsieh FJ, Chiang KC, et al. Clinical effect of a positive surgical margin after hepatectomy on survival of patients with intrahepatic cholangiocarcinoma. Drug Des Devel Ther 2014;9:163-74. [Crossref] [PubMed]
- Shinohara ET, Mitra N, Guo M, et al. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys 2008;72:1495-501. [Crossref] [PubMed]
- Jiang W, Zeng ZC, Tang ZY, et al. Benefit of radiotherapy for 90 patients with resected intrahepatic cholangiocarcinoma and concurrent lymph node metastases. J Cancer Res Clin Oncol 2010;136:1323-31. [Crossref] [PubMed]
- Hammad AY, Berger NG, Eastwood D, et al. Is Radiotherapy Warranted Following Intrahepatic Cholangiocarcinoma Resection? The Impact of Surgical Margins and Lymph Node Status on Survival. Ann Surg Oncol 2016;23:912-920. [Crossref] [PubMed]
- Lee GC, Ferrone CR, Tanabe KK, et al. Predictors of adjuvant treatment and survival in patients with intrahepatic cholangiocarcinoma who undergo resection. Am J Surg 2019;218:959-66. [Crossref] [PubMed]
- Ben-Josef E, Guthrie KA, El-Khoueiry AB, et al. SWOG S0809: A phase II intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. J Clin Oncol 2015;33:2617-22. [Crossref] [PubMed]
- Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019;20:663-73. [Crossref] [PubMed]
- Stein A, Arnold D, Bridgewater J, et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer 2015;15:564. [Crossref] [PubMed]
- Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol 2019;37:1015-27. [Crossref] [PubMed]
- Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v28-v37. [Crossref] [PubMed]
- Gallbladder cancer; intrahepatic cholangiocarcinoma; extrahepatic cholangiocarcinoma. NCCN Guidel Published online 2014:411083. Available online: http://www1.si.mahidol.ac.th/Palliative/node/30
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: Rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84-96. [Crossref] [PubMed]
- Maithel SK, Clark Gamblin T, Kamel I, et al. Multidisciplinary approaches to intrahepatic cholangiocarcinoma. Cancer 2013;119:3929-42. [Crossref] [PubMed]
- Chen YX, Zeng ZC, Tang ZY, et al. Determining the role of external beam radiotherapy in unresectable intrahepatic cholangiocarcinoma: A retrospective analysis of 84 patients. BMC Cancer 2010;10:492. [Crossref] [PubMed]
- Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2008;26:657-64. [Crossref] [PubMed]
- Goodman KA, Wiegner EA, Maturen KE, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys 2010;78:486-93. [Crossref] [PubMed]
- Jung DH, Kim MS, Cho CK, et al. Outcomes of stereotactic body radiotherapy for unresectable primary or recurrent cholangiocarcinoma. Radiat Oncol J 2014;32:163-9. [Crossref] [PubMed]
- Mahadevan A, Dagoglu N, Mancias J, et al. Stereotactic body radiotherapy (SBRT) for intrahepatic and Hilar cholangiocarcinoma. J Cancer 2015;6:1099-104. [Crossref] [PubMed]
- Sandler KA, Veruttipong D, Agopian VG, et al. Stereotactic body radiotherapy (SBRT) for locally advanced extrahepatic and intrahepatic cholangiocarcinoma. Adv Radiat Oncol 2016;1:237-43. [Crossref] [PubMed]
- Gkika E, Hallauer L, Kirste S, et al. Stereotactic body radiotherapy (SBRT) for locally advanced intrahepatic and extrahepatic cholangiocarcinoma. BMC Cancer 2017;17:781. [Crossref] [PubMed]
- Hong TS, Wo JY, Yeap BY, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2016;34:460-8. [Crossref] [PubMed]
- Tao R, Krishnan S, Bhosale PR, et al. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: A retrospective dose response analysis. J Clin Oncol 2016;34:219-26. [Crossref] [PubMed]
- Hong T, Hospital MG, Co-chair MO, et al. Radiation therapy vs. observation following gemcitabine and cisplatin for inoperable localized liver cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02200042
Cite this article as: Liu J, Malhotra G, Melstrom L, Chung V, Amini A, Chen YJ. External beam radiation treatment of intrahepatic cholangiocarcinoma: a narrative review. Dig Med Res 2021;4:12.


