Association between coeliac disease and gastrointestinal cancers: a narrative review
Introduction
Coeliac disease (CD) is a chronic immune-mediated enteropathy that affects about 1% of the Western population, with a global prevalence that is gradually increasing (1). Indeed, a recent study has reported a worldwide prevalence of 1.4% and 0.7% based on serum samples and biopsy samples, respectively (2). However, several studies have demonstrated that, due to missed or late diagnosis, the incidence of CD should be underestimated (3). The main finding in the CD is the inflammation of the small intestinal mucosal associated with a villous atrophy due to the ingestion of gluten protein products. As many autoimmune disorders, CD occurs in patients who are genetically predisposed. Indeed, the almost all the patients have HLA DR3-DQ2 and/or the DR4-DQ8 in their genome and the belief that other non-HLA locus genes such as REL, MICA, CTLA4, MMP3, MIF, TNFAIP3 (A20), NKG2D may also be involved in the disease pathogenesis is getting stronger. The main mechanism of inflammation is driven by the reaction of the intestine to gliadin fraction leading to the lamina propria and epithelium inflammation and subsequent disruption of the epithelial layer and villous atrophy. Both innate and adaptive immune response play a key role with the activation of gliadin reactive T cells, autoantibodies, intraepithelial lymphocytes, macrophages, monocyte, and dendritic cells (4). CD manifestation, when symptomatic, is characterized by typical gastrointestinal symptoms and various extra-gastrointestinal symptoms (3,5-10). The only accepted treatment is a strict gluten-free diet (GFD). Has been proven that the GFD usually improved the clinical course of the CD. Completely removing gluten is hard to achieve due to the extensive use of wheat in products and is usually associated with a decrease in quality of life (QoL) (11,12). GFD can alleviate symptoms and improve intestinal mucosal inflammation, leading to an excellent disease prognosis. However, despite a strict GFD, less than 1% of patients have CD associated with severe complications that dramatically increase mortality (13-18). Among the complications which can complicate the disease course, malignancies are the most severe. These complications have a relevant impact on the mortality, which seems to be higher than the general population (2,13,14,19-21). From a temporal point of view, the increase in overall mortality seems to be more relevant near the time of diagnosis (16-18). Indeed, it was reported by a Scottish study showed a steady decrease in mortality over time after diagnosis. In particular, the risk of malignancy declines 15 years after diagnosis (22). Despite the several studies published related to the association between CD and malignancies, many uncertainties are still present. For this reason, we aim to review the available literature and discuss the results obtained. We present this article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-51/rc).
Methods
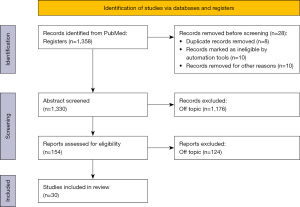
A comprehensive literature search in the PubMed database was performed on 1st of April 2022. The Prisma Flowchart is showed in Figure 1. The literature search was carried out using the following keywords: (“coeliac disease” OR “celiac disease”) AND (“cancer” OR “tumor” OR “carcinoma” OR “neoplasm”). Initially, 1,358 published papers were identified. Reference lists of relevant articles and reviews were also searched for possible studies. Titles and abstracts of all papers were scanned for potential inclusion. The research was limited to the English language. Duplicated papers, case reports, and letters were excluded. The data extraction was performed by two reviewers using standardized forms. Potential discrepancies were resolved by discussion or by a third investigator (Table 1). Finally, 30 papers were included after the authors’ revision. We identified retrospective studies (7,14,18,23-33), cohort studies (15,34-42), literature, and systematic review and meta-analysis (43-48) among these articles.
Table 1
| Items | Specification |
|---|---|
| Date of search | 1st of April, 2022 |
| Databases and other sources searched | PubMed |
| Search terms used | (“coeliac disease” OR “celiac disease”) AND (“cancer” OR “tumor” OR “carcinoma” OR “neoplasm”) |
| Timeframe | 1989–2022 |
| Inclusion and exclusion criteria | The research was limited to the English language. Duplicated papers, case reports, and letters were excluded |
| Selection process | The data extraction was performed by two reviewers using standardized forms. Potential discrepancies were resolved by discussion or by a third investigator |
| Any additional considerations, if applicable | Reference lists of relevant articles and reviews were also searched for possible studies |
Literature research
The initial research identified 1,358 studies. After the first screening, 1,204 manuscript were removed from the review. One hundred-fifty-four studies were screened, subsequently. One hundred-twenty-four papers were excluded because off-topic. The 30 studies included were divided based on the relationship between CD and different cancers. Table 2 reports the incidence of hematological cancers in patients affected by CD. Fourteen out of thirty papers described the association between CD and lymphomas. The most common was the non-Hodgkin lymphoma (NHL). Excluding the first report of 1989, the studies ranged from 2002 to 2021, analyzing 97,943 patients and reporting a mean risk of 30-time fold to develop a hematological cancer. Table 3 shows the data about the small bowel carcinoma. Fifteen studies were included. A total of 181,790 patients were analyzed from 2002 to 2020. The CD patients had 15-time fold increased risk to develop a small bowel cancer (SBC). Data regarding lower gastrointestinal (GI) are reported in Table 4. Thirteen studied were included. 203,887 patients were studied, reporting an overall risk increased of two-time fold. Table 5 underlines the risk of develop an esophageal cancer in the CD population. Twelve studies were reviewed, showing four-time fold risk of occurrence. Table 6 shows the association between CD and gastric cancer. Seven studies were analyzed. The majority of them demonstrated no correlation between the diseases. Table 7 reports the incidence of pancreatic cancer in patients affected by CD. Seven studies were included. Different results were reported. However, two-time fold increased risk of pancreatic cancer was described in the CD patients. Table 8 shows data about liver cancer. Five studies were included with a mean risk of two-time fold reported.
Table 2
| Year | Authors | Country | Study design | Sample size | Main results |
|---|---|---|---|---|---|
| 1989 | Holmes et al. | UK | Retrospective observational study | 210 | CD was correlated to NHL, increasing the risk of 42.7 time-fold (95% CI: 19.6–81.4) |
| 2002 | Askling et al. | Sweden | Prospective population-based observational study | 11,019 | Increased risk of NHL: SIR =6.3 (95% CI: 4.2–125); Increased risk of HL: SIR =4.6 (95% CI: 1.7–10) |
| 2003 | Green et al. | USA | Prospective hospital-based observational study | 381 | CD was correlated to NHL, increasing the risk of 9.1 time-fold (95% CI: 4.7–13) |
| 2004 | Card et al. | UK | Prospective population-based observational study | 869 | Peri-diagnostic period: (I) increased risk of NHL 20.94 time-fold (95% CI: 6.8–48.86); (II) increased risk of small bowel NHL 358.8 time-fold (95% CI: 74.01–1,048.34). Post-diagnostic period: (I) increased risk of NHL 5.8 time-fold (95% CI: 1.58–14.86); (II) Increased risk of small bowel NHL 40.51 time-fold (95% CI: 1.03–225.68) |
| 2004 | West et al. | UK | Retrospective population-based observational study | 4,732 | CD was correlated to NHL, increasing the risk of 4.3 time-fold (95% CI: 2.4–7.7) |
| 2006 | Viljamaa et al. | Finland | Prospective population-based observational study | 781 | CD was correlated to NHL, increasing the risk of 3.2 time-fold (95% CI: 1.0–7.5) |
| 2007 | Silano et al. | Italy | Prospective population-based observational study | 1,968 | CD was correlated to NHL, increasing the risk of 4.7 time-fold (95% CI: 2.9–7.3) |
| 2007 | Anderson et al. | UK | Retrospective population-based observational study | 490 | No increased risk of NHL |
| 2009 | Lohi et al. | Finland | Retrospective population-based observational study | 73 | CD was correlated to NHL, increasing the risk of 5.94 time-fold (95% CI: 1.41–25.04) |
| 2012 | Grainge et al. | UK | Retrospective population-based observational study | 435 | CD was correlated to NHL, increasing the risk of 12.0 time-fold (95% CI: 6.55–20.1); risk remained increased 15 years after CD diagnosis 5.15 time-fold (95% CI: 1.40–13.2) |
| 2014 | Ilus et al. | Finland | Prospective population-based observational study | 32,439 | CD was correlated to NHL, increasing the risk of 1.94 time-fold (95% CI: 1.62–2.29) |
| 2018 | Van Gils et al. | Netherlands | Retrospective population-based case-control study | – | CD was correlated to NHL, increasing the risk of 35.8 time-fold (95% CI: 27.1–47.4) |
| 2018 | Holmes et al. | UK | Prospective population-based observational study | 2,515 | CD was correlated to NHL, increasing the risk of 6.32 time-fold (2.89 to 12.00) |
| 2022 | Lebwohl et al. | Sweden | Retrospective population-based observational study | 42,241 | CD was correlated to NHL, increasing the risk of 2.2 time-fold (95% CI: 1.7–2.13) |
CD, coeliac disease; NHL, non-Hodgkin lymphoma; CI, confidence interval; SIR, standardized incidence ratio; HL, Hodgkin lymphoma.
Table 3
| Year | Authors | Country | Study design | Sample size | Main results |
|---|---|---|---|---|---|
| 2002 | Askling et al. | Sweden | Retrospective observational study | 11,019 | CD was correlated to SBC increasing the risk of 10 time-fold (95% CI: 4.4–20) |
| 2003 | Green et al. | USA | Prospective observational study | 381 | CD was correlated to SBC increasing the risk of 34 time-fold (95% CI: 24–42) |
| 2004 | Card et al. | UK | Prospective observational study | 869 | Peri-diagnostic period: increased risk of SBC 59.97 time-fold (95% CI: 1.52–334.12). Post-diagnostic period: no increased risk detected |
| 2004 | West et al. | UK | Retrospective observational study | 4,732 | Overall: increased risk of 1.95 time-fold (1.27–3.00). First year after diagnosis: increased risk of 3.31 time-fold (1.40–7.83). Beyond the first year after diagnosis: increased risk of 1.65 time-fold (0.99–2.76) |
| 2007 | Silano et al. | Italy | Prospective observational study | 1,968 | CD was correlated to SBC increasing the risk of 25 time-fold (95% CI: 8.5–51.4) |
| 2007 | Anderson et al. | UK | Retrospective observational study | 490 | CD was correlated to SBC. However, no statistically significant differences were reached |
| 2009 | Lohi et al. | Finland | Retrospective observational study | 73 | CD was correlated to SBC increasing the risk of 1.38 time-fold (95% CI: 0.60–3.14) |
| 2012 | Grainge et al. | UK | Retrospective observational study | 435 | CD was correlated to SBC increasing the risk of 11.1 time-fold (95% CI: 0.28–61.6) |
| 2012 | Elfström et al. | Sweden | Prospective observational study | – | CD was correlated to SBC increasing the risk of 2.22 time-fold (95% CI: 1.19–4.14) |
| 2014 | Ilus et al. | Finland | Retrospective observational study | 32,439 | CD was correlated to SBC increasing the risk: (I) All cases: 4.29 time-fold (95% CI: 2.83–6.24); (II) Males: 3.47 time-fold (95% CI: 1.66–6.37); (III) Females: 5.00 time-fold (95% CI: 2.91–7.99) |
| 2015 | Han et al. | China | Metanalysis | 79,365 | CD was correlated to SBC increasing the risk of 14.4 time-fold (95% CI: 5.53–37.60) |
| 2018 | Van Gils et al. | Netherlands | Retrospective case-control study |
– | CD was correlated to SBC increasing the risk of 11.9 time-fold (95% CI: 8.2–17.2) |
| 2020 | Emilsson et al. | Sweden | Retrospective observational study | 48,119 CD patients and 239,249 controls | CD was correlated to SBC increasing the risk of 3.05 time-fold (95% CI: 1.86–4.99) |
CD, coeliac disease; SBC, small bowel carcinoma; CI, confidence interval.
Table 4
| Year | Authors | Country | Study design | Sample size | Main results |
|---|---|---|---|---|---|
| 2002 | Askling et al. | Sweden | Prospective population-based observational study | 11,019 | Compared to the general population: (I) CD was correlated to colon cancer increasing the risk of 1.9 time-fold (95% CI: 1.2–2.8); (II) No statistically significant differences were reached with rectal cancer |
| 2003 | Green et al. | USA | Prospective hospital-based observational study | 381 | No statistically significant differences were reached with colon cancer |
| 2006 | Viljamaa et al. | Finland | Prospective population-based observational study | 781 | No statistically significant differences were reached with colon and rectal cancer |
| 2007 | Silano et al. | Italy | Prospective population-based observational study | 1,968 | No statistically significant differences were reached with colon cancer |
| 2008 | Goldacre et al. | UK | Retrospective hospital-based observational study | 1,997 | No statistically significant differences were reached with colon and rectal cancer |
| 2011 | Landgren et al. | USA | Retrospective hospital-based observational study | NR | No statistically significant differences were reached with colon and rectal cancer |
| 2012 | Grainge et al. | UK | Retrospective population-based observational study | 435 | No statistically significant differences were reached with colon cancer |
| 2012 | Elfström et al. | Sweden | Prospective observational study | 28,989 | 1st year of FU: (I) CD was correlated to colon cancer increasing the risk of 7.94 time-fold (95% CI: 5.21–12.1); (II) CD was correlated to rectal cancer increasing the risk of 2.57 time-fold (95% CI: 1.36–4.86). After 1 year of FU: no statistically significant differences were reached with colon and rectal cancer |
| 2014 | Ilus et al. | Finland | Retrospective observational study | 32,439 | CD was correlated to colon cancer increasing the risk of 1.35 time-fold (95% CI: 1.13–1.58). No statistically significant differences were reached with rectal cancer |
| 2014 | Volta et al. | Italy | Multicenter retrospective observational study | 1,757 | CD was correlated to colon cancer by decreasing the risk of 0.29 time-fold (95% CI: 0.07–0.45) |
| 2015 | Han et al. | China | Meta-analysis | 79,365 | No statistically significant differences were reached with colon and rectal cancer |
| 2018 | Holmes et al. | UK | Prospective population-based observational study | 2,515 | No statistically significant differences were reached with colon and rectal cancer |
| 2022 | Lebwohl et al. | Sweden | Retrospective population-based observational study | 42,241 | No statistically significant differences were reached with colon and rectal cancer |
CD, coeliac disease; CI, confidence interval; FU, follow up.
Table 5
| Year | Authors | Country | Study design | Sample size | Main results |
|---|---|---|---|---|---|
| 2002 | Askling et al. | Sweden | Prospective population-based observational study | 11,019 | CD was correlated to esophageal cancer increasing the risk of 4.2 time-fold (95% CI: 1.6–9.2) |
| 2003 | Green et al. | USA | Hospital-based prospective cohort study | 381 | CD was correlated to esophageal cancer increasing the risk of 12 time-fold (95% CI: 6.5–21) |
| 2008 | Goldacre et al. | UK | Retrospective hospital-based observational study | 1,997 | No statistically significant differences were reached with esophageal cancer |
| 2011 | Landgren et al. | USA | Retrospective hospital-based observational study | NR | CD was correlated to esophageal cancer increasing the risk of 1.86 time-fold (95% CI: 1.03–3.36) |
| 2012 | Grainge et al. | UK | Retrospective population-based observational study | 435 | No statistically significant differences were reached with esophageal cancer |
| 2012 | Elfström et al. | Sweden | Prospective observational study | 28,989 | First year of follow-up: CD was correlated to esophageal cancer increasing the risk of 6.17 time-fold (95% CI: 1.52–25.0). After 1 year of follow-up: no statistically significant differences were reached with esophageal cancer |
| 2014 | Ilus et al. | Finland | Prospective population-based observational study | 32,439 | No statistically significant differences were reached with esophageal cancer |
| 2015 | Han et al. | China | Meta-analysis | 79,365 | CD was correlated to esophageal cancer increasing the risk of 3.72 time-fold (95% CI: 1.90 –7.28) |
| 2018 | Van Gils et al. | Netherlands | Retrospective population-based, case-control study | – | No statistically significant differences were reached with esophageal adenocarcinoma. CD was correlated to esophageal squamous cell carcinoma increasing the risk of 3.5 time-fold (95% CI: 2.1–5.8) |
| 2018 | Holmes et al. | UK | Prospective population-based observational study | 2,515 | CD was correlated to esophageal cancer increasing the risk of 2.8 time-fold (95% CI: 1.03 to 6.08) |
| 2021 | Poyrazoglu et al. | Turkey | Retrospective observational study | 63 | EC patients had a significantly higher prevalence of CD (P<0.001) |
| 2022 | He et al. | UK | Prospective population-based observational study | 478,753 | CD was correlated to esophageal cancer increasing the risk of 6.89 time-fold (95% CI: 2.18-21.75) |
CD, coeliac disease; CI, confidence interval; EC, esophageal cancer.
Table 6
| Year | Authors | Country | Study design | Sample size | Main results |
|---|---|---|---|---|---|
| 2002 | Askling et al. | Sweden | Prospective population-based observational study | 11,019 | No statistically significant differences were reached with gastric cancer |
| 2006 | Viljamaa et al. | Finland | Prospective population-based observational study | 781 | No statistically significant differences were reached with gastric cancer |
| 2007 | Silano et al. | Italy | Prospective population-based observational study | 1,968 | CD was correlated to gastric cancer increasing the risk of 3.0 time-fold (95% CI: 1.3–4.9) |
| 2008 | Goldacre et al. | UK | Retrospective hospital-based observational study | 1,997 | No statistically significant differences were reached with gastric cancer |
| 2012 | Elfström et al. | Sweden | Prospective observational study | 28,989 | No statistically significant differences were reached with gastric cancer |
| 2014 | Ilus et al. | Finland | Prospective population-based observational study | 32,439 | No statistically significant differences were reached with gastric cancer |
| 2015 | Han et al. | China | Meta-analysis | 79,365 | CD was correlated to gastric cancer increasing the risk of 1.53 time-fold (95% CI: 0.96–2.44) |
| 2022 | Lebwohl et al. | Sweden | Retrospective population-based observational study | 42,241 | No statistically significant differences were reached with gastric cancer |
CD, coeliac disease; CI, confidence interval.
Table 7
| Year | Authors | Country | Study design | Sample size | Main results |
|---|---|---|---|---|---|
| 2002 | Askling et al. | Sweden | Prospective population-based observational study | 11,019 | No statistically significant differences were reached with pancreatic cancer |
| 2008 | Goldacre et al. | UK | Retrospective hospital-based observational study | 1,997 | No statistically significant differences were reached with pancreatic cancer |
| 2011 | Landgren et al. | USA | Retrospective hospital-based observational study | NR | CD was correlated to pancreatic cancer increasing the risk of 2.27 time-fold (95% CI: 1.22–4.23) |
| 2012 | Elfström et al. | Sweden | Prospective observational study | 28,989 | First year of follow-up: CD was correlated to pancreatic cancer increasing the risk of 10.7 time-fold (95% CI: 5.77–19.7). After 1 year of follow-up: No statistically significant differences were reached with pancreatic cancer |
| 2014 | Ilus et al. | Finland | Prospective population-based observational study | 32,439 | CD was correlated to pancreatic cancer decreasing the risk of 0.73 time-fold (95% CI: 0.53–0.97) |
| 2015 | Han et al. | China | Meta-analysis | 79,365 | No statistically significant differences were reached with pancreatic cancer |
| 2022 | Lebwohl et al. | Sweden | Retrospective population-based observational study | 42,241 | CD was correlated to pancreatic cancer increasing the risk of 2.30 time-fold (95% CI: 1.87–2.82) |
NR, not available; CD, coeliac disease; CI, confidence interval.
Table 8
| Year | Authors | Country | Study design | Sample size | Main results |
|---|---|---|---|---|---|
| 2002 | Askling et al. | Sweden | Prospective population-based observational study | 11,019 | CD was correlated to liver cancer increasing the risk of 2.7 time-fold (95% CI: 1.3–4.7) |
| 2012 | Elfström et al. | Sweden | Prospective observational study | 28,989 | First year of follow-up: CD was correlated to liver cancer increasing the risk of 6.05 time-fold (95% CI: 2.96–12.4). After 1 year of follow-up: CD was correlated to liver cancer increasing the risk of 1.78 time-fold (95% CI: 1.22–2.60) |
| 2014 | Ilus et al. | Finland | Prospective population-based observational study | 32,439 | No statistically significant differences were reached with liver cancer |
| 2015 | Han et al. | China | Meta-analysis | 79,365 | CD was correlated to liver cancer increasing the risk of 2.16 time-fold (95% CI: 0.94–4.96) |
| 2022 | Lebwohl et al. | Sweden | Retrospective population-based observational study | 42,241 | CD was correlated to liver cancer increasing the risk of 1.80 time-fold (95% CI: 1.44–2.25) |
CD, coeliac disease; CI, confidence interval.
Overall risk of malignancy
The association between CD and cancer has been widely investigated. A large number of studies support the association between CD and the overall risk of malignancy (7,18,23,26-29,32,37,48,49). In contrast, several studies did not show any increase in the incidence of malignancies in a patient with CD (14,28,30-32,43,50). Interestingly, Card et al. (25) and West et al. (18) reported a decreased risk of malignancy in the post-diagnostic period and an increase in the length of the follow-up. These results were later confirmed by a Swedish study involving a large number of patients, which reported a decrease in the association between CD and cancer after the first year from diagnosis. Furthermore, several studies support this theory, reporting a higher risk of malignancy, especially in the first 1–2 years after diagnosis, followed by a radical decrease in the overall risk of malignancy to the same risk of the general population (7,26,35,38). Interestingly, the decrease of the malignancy risk over time after diagnosis seems no to be relevant to hematological cancer such as NHL, as supported by Grainge et al. (27), Green et al. (28) and West et al. (18) As a matter of fact, these studies reported a higher risk of NHL in CD compared to general population even years after diagnosis.
If this decreased risk of malignancy could be related, the benefit of a GFD is still under investigation even if several studies (23,29,43,46), such as the one published by Corrao et al. (16) seems to support this hypothesis.
Together with GFD, another aspect that must be considered when analyzing the decrease of the overall risk of malignancy over time is the improvement in the diagnostic field. Indeed, a steady inflammation state of the intestinal mucosa seems to be at the base of the carcinogenic pathway, and for this reason, an early diagnosis with an immediate adhesion to a GFD could have a hinge role, as reported by the retrospective study of Eigner et al. (38).
Malignancies must be considered one of the most severe complications related to CD. Indeed, studies by Lebwohl et al. (49) and by Anderson et al. (14) reported that the risk of malignancy seems to increase together with the risk of mortality in patients with CD compared to the general population. Furthermore, in a study by Pelizzaro et al. (47) the results showed a radical increase in mortality, especially in the pediatric population.
Even if the association between CD and the overall risk of malignancies still needs to be clearly defined, an association between some specific malignancies (small bowel carcinoma and gastrointestinal lymphomas) is already established.
NHL
Association between CD and GI malignancies has been already investigated for years. Several studies demonstrated an association with a 60% increase in GI cancer in patients with CD. Indeed, it has already been proved that the association between CD and lymphomas (18,23-28,31,51), in particular gut lymphomas (25,52), such as enteropathy-associated T cell lymphoma (EATL) (53). EATL is an aggressive form of NHL of the gut, which accounts for only 5% of all gastrointestinal lymphomas (54-56) and characteristically arises when refractory CD occurs. EATL is commonly characterized by medium-sized to large lymphocytes together with component of chronic inflammatory cells. When this lymphoma is characterized by the presence of monomorphic medium-sized cells constitute a distinct entity which was classified as EATL type 2 and now is called monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL). No association between MEITL and CD has been demonstrated. EATL is usually characterized by a multifocal involvement mainly of the jejunum, and in other cases, the remaining GI tract is involved (38). Refractory CD (RCD), which can be considered a low-grade precursor lesion that clinically resembles RCD, is defined as persistent or recurrent symptoms and signs of malabsorption with villous atrophy despite a strict GFD for at least months (54,57), and it can be classified as type one and two. RCD type 1 and 2 are both characterized by lesions with no atypia of intraepithelial lymphocyte. There are some differences between the type 1 and 2, such as: the presence of normal surface T cell receptor; the expression, by intraepithelial T lymphocytes, of both CD3 and CD8; and the presence of a polyclonal pattern on T cell receptor gene rearrangement studies which can be found only in type 1 RCD. Indeed, RCD type 2 is characterized by loss of surface T cell receptor, CD3 or CD8 expression, and the presence of a monoclonal T cell receptor gene rearrangement. While type I RCD is unlikely to progress to EATL, type II may be a precursor lesion to EATL. This condition could explain why the risk of NHL is higher even years after the diagnosis of CD and the beginning of a strict GFD, as reported in several studies. Indeed, it is not clear if a strict GFD can avoid EATL development. Studies by Holmes et al. (29) and Collin et al. (58) reported a decreased risk of NHL in CD patients who altogether avoid gluten intake. The same hypothesis is supported by Silano et al. in their article, where they described a protective role of GFD in CD patients (23).
In contrast, studies published by Swinson et al. and Green et al. showed the development of intestinal lymphoma even in patients with good histological response to GFD (28,59).
This conflicting situation could be explained by the difficulty of completely avoiding intake of gluten since, nowadays, it can be found in small quantities in a lot of non-cereal food, as supported by Catassi et al. (56).
Small bowel carcinoma
Together with EATL, another cancer associated with CD is SBC. As EATL, SBC is a rare GI neoplasm which accounts for 40% of small bowel neoplasm and just 5% of all GI cancers (60).
SBC arising in CD patients is characterized by distinctive clinical, histological, pathological, and molecular features, and it is considered different from sporadic SBC or the one related to Crohn’s disease.
Indeed, even if we are talking of the same cancer, Caio et al. (42) and Vanoli et al. (39) reported that CD patients who develop SBC have a better prognosis than patients with sporadic, hereditary, or Crohn’s disease-related SBC.
In CD patients, diets rich in gluten led to a state of chronic inflammation at the level of the intestinal mucosa. Since chronic inflammation has a hinge role in the carcinogenic process (61-63), the association between a chronic immune-mediated enteropathy as CD and SBC has been widely investigated by several studies (18,23,24,28,32,37,41,44,46,47).
In 2017, Zullo et al. published a study composed of a case report and a literature review about SBC where he reported a higher risk of SBC in CD patients only in the first 2 years after diagnosis (40). In contrast with data related to NHL and, in particular, EATL, these results seem to support the hypothesis that following a strict GFD can bring advantages in silencing chronic inflammatory status and reducing the risk of developing SBC.
This hypothesis is even further supported by the higher risk of development of SBC in the pre-diagnostic period compared to the post-diagnostic period.
SBC development seems to be based on the so-called “adenoma to carcinoma” (64). In 2020, Emilsson et al. investigated this hypothesis and reported a 5.7-fold increase risk of small bowel adenomas and an increased risk of SBC in a nationwide cohort of CD patients (44).
Other GI tumors
Among all the other types of gastrointestinal tumors, there is not enough evidence to support any association with CD patients.
Even if SBC has a higher CD incidence than the general population, investigations related to colorectal cancer gave controversial results. Indeed, in 2002, Askling et al. (24) published an increased risk of colon cancer, mainly in the ascending and transverse colon, in CD patients. Controversially, almost ten years later, an Italian study conducted by Volta et al. reported a decreased risk of colon cancer in 1,757 patients with CD (33). Even so, most studies investigating this association reported no difference between CD patients and the general population.
In September 2015, Han et al. (37) published a meta-analysis that reported an association between CD patients with a higher increase in GI cancers, and among single types of tumors, esophageal cancer was found to be associated with CD.
The available results are even more controversial when focusing on other GI cancer. Indeed, tumors of the stomach, liver, pancreas, and esophagus were investigated in several studies without obtaining significant results (7,23,24,26-28,31,32,35,41).
Conclusions
Association between CD and cancer has been widely investigated for years, and the associations between CD patients and higher risk of EATL and SBC were discovered. The literature is still too controversial regarding other tumors and needs more data to analyze this topic further. The overall risk of GI cancer, apart from NHL, seems to be influenced by the temporal distance from the time of CD diagnosis, suggesting a possible correlation with GFD. However, further high-quality studies need to be published to support this hypothesis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-51/rc
Peer Review File: Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-51/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-51/coif). MDP serves as an unpaid editorial board member of Digestive Medicine Research from October 2022 to September 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dubé C, Rostom A, Sy R, et al. The prevalence of celiac disease in average-risk and at-risk Western European populations: a systematic review. Gastroenterology 2005;128:S57-67. [Crossref] [PubMed]
- Singh P, Arora A, Strand TA, et al. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018;16:823-836.e2. [Crossref] [PubMed]
- Di Sabatino A, Corazza GR. Coeliac disease. Lancet 2009;373:1480-93. [Crossref] [PubMed]
- Carreras J. Artificial Intelligence Analysis of Celiac Disease Using an Autoimmune Discovery Transcriptomic Panel Highlighted Pathogenic Genes including BTLA. Healthcare (Basel) 2022;10:1550. [Crossref] [PubMed]
- Volta U, Caio G, Stanghellini V, et al. The changing clinical profile of celiac disease: a 15-year experience (1998-2012) in an Italian referral center. BMC Gastroenterol 2014;14:194. [Crossref] [PubMed]
- Kivelä L, Kaukinen K, Lähdeaho ML, et al. Presentation of Celiac Disease in Finnish Children Is No Longer Changing: A 50-Year Perspective. J Pediatr 2015;167:1109-15.e1. [Crossref] [PubMed]
- Lebwohl B, Green PHR, Emilsson L, et al. Cancer risk in 47,241 individuals with celiac disease: a nationwide cohort study. Clin Gastroenterol Hepatol 2022;20:e111-31. [Crossref] [PubMed]
- Riznik P, De Leo L, Dolinsek J, et al. Diagnostic Delays in Children With Coeliac Disease in the Central European Region. J Pediatr Gastroenterol Nutr 2019;69:443-8. [Crossref] [PubMed]
- Parzanese I, Qehajaj D, Patrinicola F, et al. Celiac disease: From pathophysiology to treatment. World J Gastrointest Pathophysiol 2017;8:27-38. [Crossref] [PubMed]
- Van Kalleveen MW, de Meij T, Plötz FB. Clinical spectrum of paediatric coeliac disease: a 10-year single-centre experience. Eur J Pediatr 2018;177:593-602. [Crossref] [PubMed]
- Marsilio I, Canova C, D'Odorico A, et al. Quality-of-Life Evaluation in Coeliac Patients on a Gluten-Free Diet. Nutrients 2020;12:2981. [Crossref] [PubMed]
- Zingone F, Swift GL, Card TR, et al. Psychological morbidity of celiac disease: A review of the literature. United European Gastroenterol J 2015;3:136-45. [Crossref] [PubMed]
- Tio M, Cox MR, Eslick GD. Meta-analysis: coeliac disease and the risk of all-cause mortality, any malignancy and lymphoid malignancy. Aliment Pharmacol Ther 2012;35:540-51. [Crossref] [PubMed]
- Anderson LA, McMillan SA, Watson RG, et al. Malignancy and mortality in a population-based cohort of patients with coeliac disease or "gluten sensitivity". World J Gastroenterol 2007;13:146-51. [Crossref] [PubMed]
- Holmes GKT, Muirhead A. Mortality in coeliac disease: a population-based cohort study from a single centre in Southern Derbyshire, UK. BMJ Open Gastroenterol 2018;5:e000201. [Crossref] [PubMed]
- Corrao G, Corazza GR, Bagnardi V, et al. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet 2001;358:356-61. [Crossref] [PubMed]
- Ludvigsson JF, Montgomery SM, Ekbom A, et al. Small-intestinal histopathology and mortality risk in celiac disease. JAMA 2009;302:1171-8. [Crossref] [PubMed]
- West J, Logan RF, Smith CJ, et al. Malignancy and mortality in people with coeliac disease: population based cohort study. BMJ 2004;329:716-9. [Crossref] [PubMed]
- Blázquez AB, Berin MC. Microbiome and food allergy. Transl Res 2017;179:199-203. [Crossref] [PubMed]
- D'Argenio V, Casaburi G, Precone V, et al. Metagenomics Reveals Dysbiosis and a Potentially Pathogenic N. flavescens Strain in Duodenum of Adult Celiac Patients. Am J Gastroenterol 2016;111:879-90. [Crossref] [PubMed]
- Lebwohl B, Blaser MJ, Ludvigsson JF, et al. Decreased risk of celiac disease in patients with Helicobacter pylori colonization. Am J Epidemiol 2013;178:1721-30. [Crossref] [PubMed]
- Quarpong W, Card TR, West J, et al. Mortality in people with coeliac disease: Long-term follow-up from a Scottish cohort. United European Gastroenterol J 2019;7:377-87. [Crossref] [PubMed]
- Silano M, Volta U, Mecchia AM, et al. Delayed diagnosis of coeliac disease increases cancer risk. BMC Gastroenterol 2007;7:8. [Crossref] [PubMed]
- Askling J, Linet M, Gridley G, et al. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology 2002;123:1428-35. [Crossref] [PubMed]
- Card TR, West J, Holmes GK. Risk of malignancy in diagnosed coeliac disease: a 24-year prospective, population-based, cohort study. Aliment Pharmacol Ther 2004;20:769-75. [Crossref] [PubMed]
- Goldacre MJ, Wotton CJ, Yeates D, et al. Cancer in patients with ulcerative colitis, Crohn's disease and coeliac disease: record linkage study. Eur J Gastroenterol Hepatol 2008;20:297-304. [Crossref] [PubMed]
- Grainge MJ, West J, Solaymani-Dodaran M, et al. The long-term risk of malignancy following a diagnosis of coeliac disease or dermatitis herpetiformis: a cohort study. Aliment Pharmacol Ther 2012;35:730-9. [Crossref] [PubMed]
- Green PH, Fleischauer AT, Bhagat G, et al. Risk of malignancy in patients with celiac disease. Am J Med 2003;115:191-5. [Crossref] [PubMed]
- Holmes GK, Prior P, Lane MR, et al. Malignancy in coeliac disease--effect of a gluten free diet. Gut 1989;30:333-8. [Crossref] [PubMed]
- Lohi S, Mäki M, Montonen J, et al. Malignancies in cases with screening-identified evidence of coeliac disease: a long-term population-based cohort study. Gut 2009;58:643-7. [Crossref] [PubMed]
- Viljamaa M, Kaukinen K, Pukkala E, et al. Malignancies and mortality in patients with coeliac disease and dermatitis herpetiformis: 30-year population-based study. Dig Liver Dis 2006;38:374-80. [Crossref] [PubMed]
- Ilus T, Kaukinen K, Virta LJ, et al. Incidence of malignancies in diagnosed celiac patients: a population-based estimate. Am J Gastroenterol 2014;109:1471-7. [Crossref] [PubMed]
- Volta U, Vincentini O, Quintarelli F, et al. Low risk of colon cancer in patients with celiac disease. Scand J Gastroenterol 2014;49:564-8. [Crossref] [PubMed]
- Collin P, Pukkala E, Reunala T. Malignancy and survival in dermatitis herpetiformis: a comparison with coeliac disease. Gut 1996;38:528-30. [Crossref] [PubMed]
- Elfström P, Granath F, Ye W, et al. Low risk of gastrointestinal cancer among patients with celiac disease, inflammation, or latent celiac disease. Clin Gastroenterol Hepatol 2012;10:30-6. [Crossref] [PubMed]
- Landgren AM, Landgren O, Gridley G, et al. Autoimmune disease and subsequent risk of developing alimentary tract cancers among 4.5 million US male veterans. Cancer 2011;117:1163-71. [Crossref] [PubMed]
- Han Y, Chen W, Li P, et al. Association Between Coeliac Disease and Risk of Any Malignancy and Gastrointestinal Malignancy: A Meta-Analysis. Medicine (Baltimore) 2015;94:e1612. [Crossref] [PubMed]
- Eigner W, Bashir K, Primas C, et al. Dynamics of occurrence of refractory coeliac disease and associated complications over 25 years. Aliment Pharmacol Ther 2017;45:364-72. [Crossref] [PubMed]
- Vanoli A, Di Sabatino A, Furlan D, et al. Small Bowel Carcinomas in Coeliac or Crohn's Disease: Clinico-pathological, Molecular, and Prognostic Features. A Study From the Small Bowel Cancer Italian Consortium. J Crohns Colitis 2017;11:942-53. [Crossref] [PubMed]
- Zullo A, De Francesco V, Manta R, et al. A Challenging Diagnosis of Jejunal Adenocarcinoma in a Celiac Patient: Case Report and Systematic Review of the Literature. J Gastrointestin Liver Dis 2017;26:411-5. [Crossref] [PubMed]
- van Gils T, Nijeboer P, Overbeek LI, et al. Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: Consequences for follow-up: Celiac disease, lymphoma and GI carcinoma. United European Gastroenterol J 2018;6:1485-95. [Crossref] [PubMed]
- Caio G, Volta U, Ursini F, et al. Small bowel adenocarcinoma as a complication of celiac disease: clinical and diagnostic features. BMC Gastroenterol 2019;19:45. [Crossref] [PubMed]
- Marafini I, Monteleone G, Stolfi C. Association Between Celiac Disease and Cancer. Int J Mol Sci 2020;21:4155. [Crossref] [PubMed]
- Emilsson L, Semrad C, Lebwohl B, et al. Risk of Small Bowel Adenocarcinoma, Adenomas, and Carcinoids in a Nationwide Cohort of Individuals With Celiac Disease. Gastroenterology 2020;159:1686-1694.e2. [Crossref] [PubMed]
- Poyrazoglu OB, Dulger AC. Celiac disease is increased in esophageal squamous cell Carcinoma. Pak J Med Sci 2021;37:1445-50. [Crossref] [PubMed]
- Wang M, Yu M, Kong WJ, et al. Association between intestinal neoplasms and celiac disease: A review. World J Gastrointest Oncol 2021;13:1017-28. [Crossref] [PubMed]
- Pelizzaro F, Marsilio I, Fassan M, et al. The Risk of Malignancies in Celiac Disease-A Literature Review. Cancers (Basel) 2021;13:5288. [Crossref] [PubMed]
- He MM, Lo CH, Wang K, et al. Immune-Mediated Diseases Associated With Cancer Risks. JAMA Oncol 2022;8:209-19. [Crossref] [PubMed]
- Lebwohl B, Green PHR, Söderling J, et al. Association Between Celiac Disease and Mortality Risk in a Swedish Population. JAMA 2020;323:1277-85. [Crossref] [PubMed]
- Abdul Sultan A, Crooks CJ, Card T, et al. Causes of death in people with coeliac disease in England compared with the general population: a competing risk analysis. Gut 2015;64:1220-6. [Crossref] [PubMed]
- Grainge MJ, West J, Card TR, et al. Causes of death in people with celiac disease spanning the pre- and post-serology era: a population-based cohort study from Derby, UK. Am J Gastroenterol 2011;106:933-9. [Crossref] [PubMed]
- Catassi C, Fabiani E, Corrao G, et al. Risk of non-Hodgkin lymphoma in celiac disease. JAMA 2002;287:1413-9. [Crossref] [PubMed]
- O'Farrelly C, Feighery C, O'Briain DS, et al. Humoral response to wheat protein in patients with coeliac disease and enteropathy associated T cell lymphoma. Br Med J (Clin Res Ed) 1986;293:908-10. [Crossref] [PubMed]
- Sharaiha RZ, Lebwohl B, Reimers L, et al. Increasing incidence of enteropathy-associated T-cell lymphoma in the United States, 1973-2008. Cancer 2012;118:3786-92. [Crossref] [PubMed]
- Verbeek WH, Van De Water JM, Al-Toma A, et al. Incidence of enteropathy--associated T-cell lymphoma: a nation-wide study of a population-based registry in The Netherlands. Scand J Gastroenterol 2008;43:1322-8. [Crossref] [PubMed]
- Catassi C, Bearzi I, Holmes GK. Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology 2005;128:S79-86. [Crossref] [PubMed]
- Rubio-Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut 2010;59:547-57. [Crossref] [PubMed]
- Collin P, Reunala T, Pukkala E, et al. Coeliac disease--associated disorders and survival. Gut 1994;35:1215-8. [Crossref] [PubMed]
- Swinson CM, Slavin G, Coles EC, et al. Coeliac disease and malignancy. Lancet 1983;1:111-5. [Crossref] [PubMed]
- Aparicio T, Zaanan A, Svrcek M, et al. Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis 2014;46:97-104. [Crossref] [PubMed]
- Li Q, Withoff S, Verma IM. Inflammation-associated cancer: NF-kappaB is the lynchpin. Trends Immunol 2005;26:318-25. [Crossref] [PubMed]
- Kiraly O, Gong G, Olipitz W, et al. Inflammation-induced cell proliferation potentiates DNA damage-induced mutations in vivo. PLoS Genet 2015;11:e1004901. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Rampertab SD, Forde KA, Green PH. Small bowel neoplasia in coeliac disease. Gut 2003;52:1211-4. [Crossref] [PubMed]
Cite this article as: Montorsi RM, Esposito A, De Pastena M. Association between coeliac disease and gastrointestinal cancers: a narrative review. Dig Med Res 2023;6:18.