
Branched chain amino acid-to-tyrosine ratio: not only an indicator of the amino acid imbalance
Introduction
Chronic liver diseases induce various metabolic disorders. Amino acid imbalance is known to be associated with the symptom of hepatic encephalopathy, especially in patients with advanced cirrhosis (1-4). However, chronic liver diseases gradually progress over several decades (5). In a previous study (6), we showed that amino acid imbalance progresses even before leading to cirrhosis and is also associated with the severity of esophagogastric varices in patients with hepatitis C virus (HCV) infection.
We herein report an overview of the amino acid imbalance in patients with chronic liver diseases. In addition, we discuss the branched chain amino acid (BCAA)-to-tyrosine ratio (BTR) (7), which not only reflects the degree of amino acid imbalance but also is associated with various clinical aspects in patients with chronic liver diseases.
Amino acid imbalance and the BTR
Cirrhotic patients are known to show specific changes in the serum levels of amino acids, which are characterized by a decrease in BCAAs (valine, leucine and isoleucine) and increase in aromatic amino acids (AAAs). Thus, a low BCAA-to-AAA ratio (Fisher’s ratio) is generally recognized to reflect amino acid imbalance (2,3). Hyperammonemia and hepatic encephalopathy are well-known events associated with amino acid imbalance, and the improvement of hyperammonemia with BCAA treatment has been reported in various studies (8-10). Currently, in the Japanese guidelines for the management of liver cirrhosis, the supplementation of BCAA is described as a standard treatment option for hepatic encephalopathy (11,12).
Fischer’s ratio is known to be a well-established indicator for assessing the amino acid imbalance and hepatic encephalopathy. However, a total amino acid analysis is laborious and costly to perform, so the BTR was proposed (7). The BTR correlates well with Fischer’s ratio and is commonly used in Japan to evaluate the amino acid imbalance (13-16). In a previous study (6), we investigated the relationship between the liver fibrosis stage and BTR in HCV-positive patients and showed that the BTR gradually decreased in line with the severity of liver fibrosis, even during non-cirrhotic stages (6). Therefore, the amino acid imbalance occurs at an early stage of chronic liver disease, before changes in the general indicators of the hepatic function, such as prothrombin time and bilirubin, are observed. Michitaka et al. (16) showed that the tyrosine level increased during non-cirrhotic stages, whereas a decrease in BCAA levels was mainly found in the cirrhotic stage. Thus, an increase in AAAs rather than a decrease in BCAAs was suggested to relate to the early symptom of amino acid imbalance. Although the BTR is not a pure fibrosis marker, it appears to be an indicator that widely reflects the pathophysiology progression of chronic liver diseases.
BTR in cirrhotic patients
BTR and skeletal muscles
In cirrhotic patients, ammonia clearance in the liver is decreased, and the skeletal muscle plays an important role in ammonia metabolism (17,18) (Figure 1). In skeletal muscle cells, BCAAs are consumed for the clearance of ammonia, so supplementation of BCAAs may help control hyperammonemia.
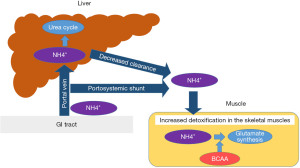
As the skeletal muscle plays a significant role in ammonia detoxification in cirrhotic patients, reduced muscle mass can lead to hyperammonemia. However, liver cirrhosis is known to be associated with a high risk of a decreased muscle mass (sarcopenia) (19,20). In addition, it is assumed that hyperammonemia itself causes the muscle volume loss, thus further exacerbating the faulty ammonia clearance. BCAA supplementation is suggested to increase the muscle mass (21,22), and the administration of BCAAs is expected to cease such a vicious cycle by not only improving the amino acid imbalance but also increasing the muscle mass.
A low BTR is not only an indicator of amino acid imbalance, which is caused by the metabolism of BCAAs and AAAs, but also a significant predictive factor for a decreased skeletal muscle mass (23). Thus, BCAA administration is considered particularly beneficial for such patients.
BTR and albumin production
Cirrhotic patients frequently develop malnutrition, which may be related to a poor prognosis, and those with a low level of serum albumin (≤3.5 g/dL) are considered to have “protein malnutrition” (24,25). In addition to improving the amino acid imbalance, BCAA administration is known to increase the serum albumin level (26,27). BCAAs have been found to not only be useful as a material for albumin synthesis but also function as a physiologically active substance that promotes albumin synthesis in hepatocytes (28). BCAA administration was suggested to activate the signal pathway of the mammalian/mechanistic target of rapamycin (mTOR) and stimulate albumin synthesis (29). In addition, BCAA metabolites, including branched-chain α-keto acids (BCKA), β-hydroxy-β-methyl butyrate (HMB) and glutamate, also activate mTOR pathways and contribute to protein synthesis (30).
Long-term administration of BCAAs has been shown to improve serum albumin levels (26,27). In Japan, BCAA granules are commonly used to improve the hypoalbuminemia in cirrhotic patients. However, even among patients with compensated cirrhosis, there are some who develop the mild amino acid imbalance, and early administration of BCAAs to such patients has been shown to prevent the decrease in serum albumin values (31,32). A decreased BTR was reported to not only relate to the presence of hypoalbuminemia but also to be a useful predictive marker for the development of hypoalbuminemia (33).
BTR and insulin resistance
Dysregulation of glucose metabolism is frequently observed in patients with chronic HCV infection (34). In patients with chronic liver diseases, BCAAs are suggested to function in various organs and improve the insulin resistance (28). In the skeletal muscle, BCAA treatment accelerates the glucose uptake through the activation of two pathways: the phosphatidylinositol 3-kinase (PI3K)-protein kinase C (PKC) pathway and the peroxisome proliferator-activated receptor α (PPARα) pathway. In the adipose tissue, BCAA administration activates the Akt-mTOR signal pathway, and the glucose uptake increases. In the liver, BCAA treatment increases the glucose uptake through the activation of the liver X receptor α (LXRα)/sterol regulatory element binding protein-1c (SREBP1-c) pathway and the PPARα pathway (28). A randomized, controlled, crossover trial showed that BCAA treatment decreased the HbA1c values in HCV-infected patients with insulin resistance (35). Thus, amino acid imbalance, represented by a low BTR, is suggested to closely relate to insulin resistance.
BTR and invasive treatments
As mentioned above, oral administration of BCAAs has been used to treat protein malnutrition (36). In the endoscopic treatment of gastroesophageal varices, dietary restriction is generally included in the treatment protocol, but it may deteriorate the nutritional status. In patients with liver cirrhosis, oral intake of a late evening snack (LES) is recognized as an effective nutritional therapy (37,38). BCAA treatment has been suggested to provide some benefits to patients who receive invasive treatments (39).
We suspected that BCAA administration might exert some favorable effects on the maintenance of the nutritious status after endoscopic treatment and assessed the nutrition of cirrhotic patients who received endoscopic therapy for varices (40). BCAA alone was effective in preventing a decrease in the albumin value, whereas BCAA-containing nutritional supplement showed favorable effects on protein and energy malnutrition (40). These findings suggest that supplementing calories may help BCAAs exert their effects on improving the nutritious status.
However, despite similar results having been reported elsewhere as well (41,42), we should pay attention to the fact that the BTR in patients with esophageal varices, particularly in those with high-risk varices, tends to be low (6). Therefore, the nutritious benefits provided by BCAA administration may be associated with the enrolled patients having a low BTR. It is therefore still necessary to determine whether or not the BTR is associated with the efficacy of BCAA treatment in patients receiving invasive treatments.
BTR and the treatment efficacy of BCAA administration
Long-term BCAA administration has been shown to suppress the risk of all fatal events, such as the development of liver cancer, bleeding of esophagogastric varices, and progression of liver failure (encephalopathy, icterus and ascites) (43). BCAA administration showed particularly favorable effects on patients with HCV-related cirrhosis compared with patients with other etiologies (43,44). It was also shown that the administration of BCAA reduced the incidence of liver cancer in patients with HCV-related cirrhosis and obesity, whose body mass index (BMI) was ≥25 kg/m2 (44).
As mentioned above, BCAA was suggested to increase muscle mass and contribute to lower serum ammonia levels (21,22). The presence of sarcopenia, a decreased muscle mass, is known to lead to several adverse effects and worsen the prognosis of liver disease; however, BCAA treatment was suggested to exert favorable effects on cirrhotic patients with sarcopenia (45,46). Therefore, BCAA administration may improve the prognosis through various mechanisms in addition to the correction of hyperammonemia and hypoalbuminemia.
It should be noted that undue BCAA administration may exacerbate nitrogen overload and deteriorate hyperammonemia (18). In addition, a recent study suggested that excessive BCAA administration might increase the risk of cancer development (47), so unnecessary administration should be avoided. In the current guideline of the European Society for Clinical Nutrition and Metabolism (48), BCAA therapy is considered to improve the event-free survival and quality of life in advanced cirrhotic patients, but routine administration to cirrhotic patients is not recommended.
In a previous study (49), Ishikawa et al. assessed the associations of the BTR with cirrhosis-related events (death, worsening of esophageal and/or gastric varices, hepatocellular carcinoma, and liver failure). They also compared the event-free survival between patients with BTR ≥4 and BTR <4, and a low BTR was suggested to be a predictive marker of undesired cirrhosis-related events and a poor prognosis. The BTR was also suggested to be associated with the treatment efficacy and prognosis of patients with hepatocellular carcinoma (50,51).
Hiraoka et al. assessed the usefulness of the newly developed albumin-bilirubin (ALBI) score as a possible alternative method of determining the BTR in patients with treatment-naïve hepatocellular carcinoma (14). They showed that a high ALBI score (≥−2.588) was a predictor of a low BTR (≤4.4), suggesting that advanced cirrhotic patients with a modified ALBI of at least 2b (≥−2.27) might be regarded as having an amino acid imbalance.
Therefore, patients with an amino acid imbalance and a decreased BTR may effectively obtain clinical benefits from BCAA treatment (31,32,43). The BTR, which was originally developed as an inexpensive and easily measurable index of the amino acid imbalance, may be useful for determining the indication of BCAA treatment. However, in previous reports, cut-off values ranging from 4.0 to 5.0 were used to define “low BTR”, so a further study to clarify the optimal cut-off value in order to determine the indication of BCAA administration will be needed. In addition, it would be interesting to investigate the associations of the BTR values with non-hepatic clinical characteristics, such as sex, age and ethnicity.
Conclusions
BCAA treatment is suggested to suppress cirrhosis-related adverse events and improve the prognosis in patients with advanced cirrhosis (52), although such benefits are not directly specified in the current guidelines.
The BTR was originally reported as an inexpensive and easily measurable indicator for amino acid imbalance. However, a low BTR is suggested to reflect various pathophysiological disorders of chronic liver diseases (Table 1). In addition, the BTR may also be an index for determining patients who are expected to benefit from BCAA therapy. Determining the optimal cut-off value of the BTR would allow appropriate and early supplementation of BCAA to cirrhotic patients.
Table 1
| Clinical features | Findings | Reference |
|---|---|---|
| Amino acid imbalance | BTR correlates with Fisher’s ratio and can be an indicator of the amino acid imbalance | (6,7,13-16) |
| Liver fibrosis | BTR decreases in line with the progression of liver fibrosis | (6) |
| Esophagogastric varices | BTR decreases in line with the severity of varices. In particular, patients with high-risk varices show a low BTR | (6) |
| Sarcopenia | A low BTR is a risk factor for the presence of sarcopenia | (23) |
| Hypoalbuminemia | A low BTR is a risk factor for the presence of hypoalbuminemia | (28,31,32) |
| Insulin resistance | A low BTR is suggested to relate to insulin resistance in patients with chronic liver diseases, particularly in HCV-infected patients | (28,35) |
| Overall cirrhosis-related events | A low BTR was suggested to be a predictive marker for undesired cirrhosis-related events and a poor prognosis | (49) |
| Hepatocellular carcinoma | The BTR was suggested to be associated with the treatment efficacy and prognosis of patients with hepatocellular carcinoma | (50,51) |
BTR, branched chain amino acid-to-tyrosine ratio; HCV, hepatitis C virus.
Acknowledgments
Authors thank Higuchi Y, Kanazawa N, Fujii S, Kido H, and Shimoji Y (Hyogo Medical University) for their technical and secretarial assistance.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-29/coif). HE serves as an unpaid editorial board member of Digestive Medicine Research from November 2021 to October 2023. HS serves as an unpaid editorial board member of Digestive Medicine Research from August 2022 to July 2024. HI received research grants from Otsuka Pharmaceutical Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kurpad AV. 90th Anniversary Commentary: Amino Acid Imbalances: Still in the Balance. J Nutr 2018;148:1647-9. [Crossref] [PubMed]
- James JH, Ziparo V, Jeppsson B, et al. Hyperammonaemia, plasma aminoacid imbalance, and blood-brain aminoacid transport: a unified theory of portal-systemic encephalopathy. Lancet 1979;2:772-5. [Crossref] [PubMed]
- Bernardini P, Fischer JE. Amino acid imbalance and hepatic encephalopathy. Annu Rev Nutr 1982;2:419-54. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol 2019;70:172-93. [Crossref]
- Ginès P, Krag A, Abraldes JG, et al. Liver cirrhosis. Lancet 2021;398:1359-76. [Crossref] [PubMed]
- Enomoto H, Sakai Y, Aizawa N, et al. Association of amino acid imbalance with the severity of liver fibrosis and esophageal varices. Ann Hepatol 2013;12:471-8. [Crossref] [PubMed]
- Horst D, Grace ND, Conn HO, et al. Comparison of dietary protein with an oral, branched chain-enriched amino acid supplement in chronic portal-systemic encephalopathy: a randomized controlled trial. Hepatology 1984;4:279-87. [Crossref] [PubMed]
- Dam G, Aamann L, Vistrup H, et al. The role of Branched Chain Amino Acids in the treatment of hepatic Encephalopathy. J Clin Exp Hepatol 2018;8:448-51. [Crossref] [PubMed]
- Gluud LL, Dam G, Borre M, et al. Oral branched-chain amino acids have a beneficial effect on manifestations of hepatic encephalopathy in a systematic review with meta-analyses of randomized controlled trials. J Nutr 2013;143:1263-8. [Crossref] [PubMed]
- Gluud LL, Dam G, Les I, et al. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev 2017;5:CD001939. [Crossref] [PubMed]
- Yoshiji H, Nagoshi S, Akahane T, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2020. Hepatol Res 2021;51:725-49. [Crossref] [PubMed]
- Yoshiji H, Nagoshi S, Akahane T, et al. Evidence-based clinical practice guidelines for Liver Cirrhosis 2020. J Gastroenterol 2021;56:593-619. [Crossref] [PubMed]
- Azuma Y, Maekawa M, Kuwabara Y, et al. Determination of branched-chain amino acids and tyrosine in serum of patients with various hepatic diseases, and its clinical usefulness. Clin Chem 1989;35:1399-403. [Crossref] [PubMed]
- Hiraoka A, Kato M, Marui K, et al. Easy clinical predictor for low BCAA to tyrosine ratio in chronic liver disease patients with hepatocellular carcinoma: Usefulness of ALBI score as nutritional prognostic marker. Cancer Med 2021;10:3584-92. [Crossref] [PubMed]
- Tada T, Kumada T, Toyoda H, et al. Impact of Branched-Chain Amino Acid Granule Therapy in Patients with Hepatocellular Carcinoma Who Have Normal Albumin Levels and Low Branched-Chain Amino Acid to Tyrosine Ratios. Nutr Cancer 2019;71:1132-41. [Crossref] [PubMed]
- Michitaka K, Hiraoka A, Kume M, et al. Amino acid imbalance in patients with chronic liver diseases. Hepatol Res 2010;40:393-8. [Crossref] [PubMed]
- Di Cola S, Nardelli S, Ridola L, et al. Ammonia and the Muscle: An Emerging Point of View on Hepatic Encephalopathy. J Clin Med 2022;11:611. [Crossref] [PubMed]
- Holeček M. Branched-chain amino acid supplementation in treatment of liver cirrhosis: Updated views on how to attenuate their harmful effects on cataplerosis and ammonia formation. Nutrition 2017;41:80-5. [Crossref] [PubMed]
- Tandon P, Montano-Loza AJ, Lai JC, et al. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol 2021;75:S147-62. [Crossref] [PubMed]
- Bunchorntavakul C, Reddy KR. Review article: malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment Pharmacol Ther 2020;51:64-77. [Crossref] [PubMed]
- Hernández-Conde M, Llop E, Gómez-Pimpollo L, et al. Adding Branched-Chain Amino Acids to an Enhanced Standard-of-Care Treatment Improves Muscle Mass of Cirrhotic Patients With Sarcopenia: A Placebo-Controlled Trial. Am J Gastroenterol 2021;116:2241-9. [Crossref] [PubMed]
- Bai GH, Tsai MC, Tsai HW, et al. Effects of branched-chain amino acid-rich supplementation on EWGSOP2 criteria for sarcopenia in older adults: a systematic review and meta-analysis. Eur J Nutr 2022;61:637-51. [Crossref] [PubMed]
- Nishikawa H, Enomoto H, Ishii A, et al. Development of a simple predictive model for decreased skeletal muscle mass in patients with compensated chronic liver disease. Hepatol Res 2017;47:1223-34. [Crossref] [PubMed]
- Tajika M, Kato M, Mohri H, et al. Prognostic value of energy metabolism in patients with viral liver cirrhosis. Nutrition 2002;18:229-34. [Crossref] [PubMed]
- Traub J, Reiss L, Aliwa B, et al. Malnutrition in Patients with Liver Cirrhosis. Nutrients 2021;13:540. [Crossref] [PubMed]
- Marchesini G, Bianchi G, Merli M, et al. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology 2003;124:1792-801. [Crossref] [PubMed]
- Kitajima Y, Takahashi H, Akiyama T, et al. Supplementation with branched-chain amino acids ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat accumulation in the skeletal muscles of patients with liver cirrhosis. J Gastroenterol 2018;53:427-37. [Crossref] [PubMed]
- Kawaguchi T, Izumi N, Charlton MR, et al. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology 2011;54:1063-70. [Crossref] [PubMed]
- Hara K, Yonezawa K, Weng QP, et al. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem 1998;273:14484-94. [Crossref] [PubMed]
- Tajiri K, Shimizu Y. Branched-chain amino acids in liver diseases. Transl Gastroenterol Hepatol 2018;3:47. [Crossref] [PubMed]
- Ishikawa T. Early administration of branched-chain amino acid granules. World J Gastroenterol 2012;18:4486-90. [Crossref] [PubMed]
- Nishiguchi S, Habu D. Effect of oral supplementation with branched-chain amino acid granules in the early stage of cirrhosis. Hepatol Res 2004;30S:36-41. [Crossref] [PubMed]
- Suzuki K, Suzuki K, Koizumi K, et al. Measurement of serum branched-chain amino acids to tyrosine ratio level is useful in a prediction of a change of serum albumin level in chronic liver disease. Hepatol Res 2008;38:267-72. [Crossref] [PubMed]
- Arrese M, Riquelme A, Soza A. Insulin resistance, hepatic steatosis and hepatitis C: a complex relationship with relevant clinical implications. Ann Hepatol 2010;9:112-8. [Crossref] [PubMed]
- Takeshita Y, Takamura T, Kita Y, et al. Beneficial effect of branched-chain amino acid supplementation on glycemic control in chronic hepatitis C patients with insulin resistance: implications for type 2 diabetes. Metabolism 2012;61:1388-94. [Crossref] [PubMed]
- Fukui A, Kawabe N, Hashimoto S, et al. Switching from branched-chain amino acid granules to branched-chain amino acid-enriched nutrient improves the branched-chain amino acid-to-tyrosine ratio in patients with cirrhosis with hypoalbuminemia: a prospective study. Eur J Gastroenterol Hepatol 2020;32:501-6. [Crossref] [PubMed]
- Maki H, Yamanaka-Okumura H, Katayama T, et al. Late evening snacks with branched-chain amino acids improve the Fischer ratio with patients liver cirrhosis at fasting in the next morning. Clin Nutr ESPEN 2019;30:138-44. [Crossref] [PubMed]
- Chen CJ, Wang LC, Kuo HT, et al. Significant effects of late evening snack on liver functions in patients with liver cirrhosis: A meta-analysis of randomized controlled trials. J Gastroenterol Hepatol 2019;34:1143-52. [Crossref] [PubMed]
- Nishikawa H, Osaki Y, Iguchi E, et al. The effect of long-term supplementation with branched-chain amino acid granules in patients with hepatitis C virus-related hepatocellular carcinoma after radiofrequency thermal ablation. J Clin Gastroenterol 2013;47:359-66. [Crossref] [PubMed]
- Sakai Y, Iwata Y, Enomoto H, et al. Two randomized controlled studies comparing the nutritional benefits of branched-chain amino acid (BCAA) granules and a BCAA-enriched nutrient mixture for patients with esophageal varices after endoscopic treatment. J Gastroenterol 2015;50:109-18. [Crossref] [PubMed]
- Yoshida R, Yagi T, Sadamori H, et al. Branched-chain amino acid-enriched nutrients improve nutritional and metabolic abnormalities in the early post-transplant period after living donor liver transplantation. J Hepatobiliary Pancreat Sci 2012;19:438-48. [Crossref] [PubMed]
- Hanai T, Shiraki M, Imai K, et al. Late Evening Snack with Branched-Chain Amino Acids Supplementation Improves Survival in Patients with Cirrhosis. J Clin Med 2020;9:1013. [Crossref] [PubMed]
- Muto Y, Sato S, Watanabe A, et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol 2005;3:705-13. [Crossref] [PubMed]
- Muto Y, Sato S, Watanabe A, et al. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol Res 2006;35:204-14. [Crossref] [PubMed]
- Singh Tejavath A, Mathur A, Nathiya D, et al. Impact of Branched Chain Amino Acid on Muscle Mass, Muscle Strength, Physical Performance, Combined Survival, and Maintenance of Liver Function Changes in Laboratory and Prognostic Markers on Sarcopenic Patients With Liver Cirrhosis (BCAAS Study): A Randomized Clinical Trial. Front Nutr 2021;8:715795. [Crossref] [PubMed]
- Ismaiel A, Bucsa C, Farcas A, et al. Effects of Branched-Chain Amino Acids on Parameters Evaluating Sarcopenia in Liver Cirrhosis: Systematic Review and Meta-Analysis. Front Nutr 2022;9:749969. [Crossref] [PubMed]
- Ericksen RE, Lim SL, McDonnell E, et al. Loss of BCAA Catabolism during Carcinogenesis Enhances mTORC1 Activity and Promotes Tumor Development and Progression. Cell Metab 2019;29:1151-1165.e6. [Crossref] [PubMed]
- Bischoff SC, Bernal W, Dasarathy S, et al. ESPEN practical guideline: Clinical nutrition in liver disease. Clin Nutr 2020;39:3533-62. [Crossref] [PubMed]
- Ishikawa T, Imai M, Ko M, et al. Evaluation of the branched-chain amino acid-to-tyrosine ratio prior to treatment as a prognostic predictor in patients with liver cirrhosis. Oncotarget 2017;8:79480-90. [Crossref] [PubMed]
- Eso Y, Nakano S, Mishima M, et al. Branched-chain amino acid to tyrosine ratio is an essential pre-treatment factor for maintaining sufficient treatment intensity of lenvatinib in patients with hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2020;27:913-21. [Crossref] [PubMed]
- Ishikawa T. Branched-chain amino acids to tyrosine ratio value as a potential prognostic factor for hepatocellular carcinoma. World J Gastroenterol 2012;18:2005-8. [Crossref] [PubMed]
- Hanai T, Nishimura K, Miwa T, et al. Usefulness of nutritional therapy recommended in the Japanese Society of Gastroenterology/Japan Society of Hepatology evidence-based clinical practice guidelines for liver cirrhosis 2020. J Gastroenterol 2021;56:928-37. [Crossref] [PubMed]
Cite this article as: Enomoto H, Nishimura T, Aizawa N, Takashima T, Ikeda N, Yuri Y, Fujiwara A, Yoshihara K, Yoshioka R, Kawata S, Ota S, Nakano R, Shiomi H, Iijima H. Branched chain amino acid-to-tyrosine ratio: not only an indicator of the amino acid imbalance. Dig Med Res 2023;6:1.


