
Perioperative veno-venous extracorporeal membrane oxygenation for facilitation of bronchogastric fistula repair following Ivor-Lewis oesophagectomy—case report
Introduction
A bronchogastric fistula (BGF) is one of the most devastating complications after an oesophagectomy, with potentially life-threatening respiratory complications that are complex to manage from both a surgical and critical care perspective. Fortunately, they are rare, with a reported prevalence of 0.4% to 3.9% (1). This is the first reported case in the southern hemisphere of veno-venous extracorporeal membrane oxygenation (VV-ECMO) being used as a bridge for surgical correction of a BGF post-oesophagectomy. VV-ECMO in this context allows concomitant management of adult respiratory distress syndrome (ARDS) that is reversible but temporarily unmanageable on a mechanical ventilator, whilst still allowing ultraprotective ventilatory settings (1). There is evidence to suggest that airway maintenance with lower tidal volumes and plateau pressure reduce output and improve healing of broncho-pleural fistulae (2). In the context of BGF repair, it is theoretically possible these settings also contribute towards protection of repairs/anastomoses from barotrauma, although there is no good evidence base for this.
To the best of our knowledge, there are only two case reports of VV-ECMO as a peri-operative rescue strategy to facilitate surgical repair of BGF, and one case where VV-ECMO was used for support for ongoing respiratory failure following repair. Okuyama and colleagues describe a 72-year-old male with a BGF requiring VV-ECMO for perioperative management; he was decannulated 150 hours post-operatively and was weaned off mechanical ventilation on post-operative day (POD) 41 (3). Due to the age of the article and the inability to obtain a translation from the foreign language it was written in, no further information was obtainable outside of the abstract. The second case only briefly mentions the use of ECMO to facilitate surgical repair, with discharge from intensive care unit (ICU) on POD 37 and discharge from hospital on POD 56 (4). There are no other further details discussed in this case series. As mentioned, the third report describes the successful use of VV-ECMO as a rescue therapy for ongoing hypoxia following surgical repair of a BGF, but it was not used in the pre-operative period (5).
Hence, we present the third reported case in the world of peri-operative VV-ECMO to facilitate surgical repair of post-oesophagectomy BGF, and the first reported case in the southern hemisphere. Potentially due to his age and lack of comorbidity, our patient has the earliest reported discharge from ICU and the surgical ward. Together, these reports highlight that VV-ECMO can be an extremely useful tool to help address the complex surgical and critical care challenges inherent in a post-oesophagectomy BGF. We present the following article in accordance with the CARE reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-78/rc).
Case presentation
A 47-year-old male was diagnosed with oesophageal adenocarcinoma after presenting with a 10-week history of progressive dysphagia, odynophagia and epigastric discomfort. His background was type 2 diabetes mellitus and gout. He had stopped smoking 12 years prior.
He received four cycles of neoadjuvant FLOT (Fluorouracil, Leucovorin, Oxaliplatin and Docetaxel) and proceeded to an open Ivor Lewis oesophagectomy. Both the procedure and early recovery were uncomplicated and the patient was discharged POD 8. At routine clinic follow-up the following week, he remained well. On POD 13, the patient presented to the emergency department for coughing and dyspnoea and rapidly deteriorated into type 1 respiratory failure. At intubation, a significant air leak was noted, and the resultant bronchoscopy performed showed a defect in the proximal left main bronchus, with an endoscopy confirming the presence of a BGF.
Despite being septic with respiratory failure from ongoing airway soiling, he had a period of stability that allowed transfer by air to the nearest quaternary hospital (>1,300 km). Shortly after arrival, the patient deteriorated further with a significant air leak and inability to adequately ventilate via endotracheal tube. A dual-lumen tube was placed allowing for differential lung ventilation with separate ventilators for left and right. However, ongoing respiratory failure despite maximal and the potential requirement to access the right pleural cavity for surgical repair prompted the establishment of VV-ECMO. After establishment of ECMO in the cath-lab by the cardiothoracic surgeon, the FiO2 and tidal volume requirements were reduced to ultraprotective ventilation in the setting of air leak and evolving ARDS. The patient was transferred directly to theatre for a combined operation with cardiothoracic and upper gastrointestinal (GI) surgeons, undergoing a re-do right thoracotomy, primary repair of the gastric defect, primary repair of the bronchial defect then an intercostal muscle flap fixed to the bronchus with BioGlue.
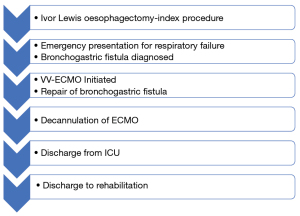
Decannulation of ECMO occurred 10 days following the redo procedure as oxygenation progressively improved with mechanical ventilation. A tracheostomy was placed to facilitate transition to ward-based management. The patient was discharged to rehabilitation 40 days post-repair and then subsequently home with no significant morbidity. He recommenced his usual employment six months after the fistula repair. Figure 1 displays a concise overview of the timeline of aforementioned clinical course.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Though there is no thorough understanding of the aetiology and formation of BGF post operatively, the predisposing factors leading up to its formation are well agreed upon. One such contributing factor is the devascularisation of bronchial arteries/arterioles during the process of dissection resulting in ischaemic portions of the trachea or bronchi (particularly during lymph node dissection), thereby increasing the risk of mucosal breakdown (6). Furthermore, there may be more direct methods of injury such as that from electrocautery or handling. Another risk factor is that of an anastomotic leak and the resultant significant inflammation within the mediastinum creating an optimal environment for fistula formation (7). Given the timeframe and acuity of the presented case, it is likely that a combination of all the aforementioned factors resulted in the complication for this patient rather one particular risk factor, as there were no intraoperative complications in the index procedure. Due to the language limitations of the article by Okuyama et al. (3) and the lack of detail with regards to the ECMO case in the case series by Lambertz et al. (4), it is difficult to analyse the strengths and limitations of said articles and therefore contrast the management principles and clinical course. Despite this, cases like the currently presented one display the true potential of ECMO in perioperative management of BGF. Trials such as the CESAR Trial (8) have shown that VV-ECMO in the management of severe ARDS can significantly improve survival without severe disability and we prove that there is no reason we can’t extrapolate this benefit to the management of a BGF. Due to its relative infrequency, no such data exists (nor is there likely to ever be high-quality evidence) for ECMO in BGF, however as ECMO continues to develop and be taken up in more facilities worldwide that there is a role in rescue of complex post-operative complications such as BGF in select patients. We have displayed that ECMO provides a not only feasible, but effective way of managing the complicated airway management associated with BGFs.
Conclusions
The case presented here is, currently, a one-of-a-kind modern, and detailed report of the management of BGF with perioperative ECMO support. The contribution of cases such as the present one to the limited armamentarium of BGF management literature is invaluable. We have displayed that with prompt transfer of a patient to a high volume, specialised quaternary centre can provide complex but ultimately lifesaving management for a complication that can have a mortality rate of up to 60% (6). We anticipate that this relatively novel management principle will only be utilised more frequently and thereby will help contribute to our understanding of how to manage this rare but devastating complication or in BGF of other aetiology.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-78/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-78/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang C, Li C, Yang X, et al. The classification and treatment strategies of post-esophagectomy airway-gastric fistula. J Thorac Dis 2020;12:3602-10. [Crossref] [PubMed]
- Shekar K, Foot C, Fraser J, et al. Bronchopleural fistula: an update for intensivists. J Crit Care 2010;25:47-55. [Crossref] [PubMed]
- Okuyama M, Suzuki H, Saito R, et al. A Successful Case of Surgery using Extracorporeal Membrane Oxygenation for Reconstructed Gastric Tube Bronchial Fistula After Operation of Esophageal Cancer. Nippon Shokaki Geka Gakkai zasshi 2000;33:102-6.
- Lambertz R, Hölscher AH, Bludau M, et al. Management of Tracheo- or Bronchoesophageal Fistula After Ivor-Lewis Esophagectomy. World J Surg 2016;40:1680-7. [Crossref] [PubMed]
- Douin DJ, Tran TT. Bronchoesophageal Fistula Requiring Venovenous ECMO After Minimally Invasive Esophagectomy. J Cardiothorac Vasc Anesth 2020;34:2727-30. [Crossref] [PubMed]
- Bartels HE, Stein HJ, Siewert JR. Tracheobronchial lesions following oesophagectomy: prevalence, predisposing factors and outcome. Br J Surg 1998;85:403-6. [Crossref] [PubMed]
- Schweigert M, Dubecz A, Beron M, et al. Management of anastomotic leakage-induced tracheobronchial fistula following oesophagectomy: the role of endoscopic stent insertion. Eur J Cardiothorac Surg 2012;41:e74-80. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
Cite this article as: Prasad A, Frankel A, Cole C, Thomson I. Perioperative veno-venous extracorporeal membrane oxygenation for facilitation of bronchogastric fistula repair following Ivor-Lewis oesophagectomy—case report. Dig Med Res 2022;5:46.


