Knowledge and surgical strategies for successful laparoscopic caudate lobectomy: a narrative review
Introduction
In the last years, many reports have demonstrated the feasibility and safety of laparoscopic liver resections (LLRs), which have been adopted in many centres worldwide, providing excellent short- and long-term results comparable to open liver resections (1,2).
Consequently, indications for LLRs have increased, including a wide variety of benign and malignant lesions. Nonetheless, the posterosuperior segments (I, IVa, VII, VIII) are still considered technically difficult locations, requiring expertise and advanced technical skills (3,4).
Caudate lobe (CL) (Couinaud segment I or Spiegel’s lobe) is also called dorsal liver and is located posteriorly, below the hepatic hilum, very close to the ductal and portal bifurcation and the hepatic arteries and above the inferior vena cava (IVC). A laparoscopic caudate lobectomy (LCL) can be associated with resection of other segments for oncological reasons, such as right or left hemi-hepatectomy for Klatskin’s tumours. In addition, isolated laparoscopic caudate lobectomy (iLCL) is indicated for some symptomatic benign or malignant (primary or secondary) lesions arising in segment I. Some reports address the results of posterosuperior LLRs compared with the anterolateral ones, but few papers are exclusively focused on iLCL, which accounted for only 2.9% of 102 LLRs enrolled in a prospective Japanese registry (5,6). iLCL has been described in some case series and case reports from single institutions; however, a real standardization of the laparoscopic approach to the dorsal liver, with an appraisal of the technical skills and technology needed, is still lacking (7-12). Furthermore, precise understanding of segment I surgical anatomy and detailed description of the right border of the caudate process is crucial in the definition of an actual “anatomical resection of the caudate lobe”. As a result, we present this narrative review of the literature of the last fifteen years, analysing the best surgical strategies for a successful laparoscopic caudate lobe resection in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-2/rc).
Methods
An electronic literature search of the PubMed, Web of Science, and Cochrane databases was performed using the Boolean operators AND, OR, NOT for the combination of keywords: “Laparoscopic”, “Hepatectomy”, “Liver resection”, “Caudate lobe”, or “Segment1”. All articles published from 1st January 2006 until 31st November 2021 were included. All English language articles related to LLRs were analysed. The references of selected papers were also screened to identify additional publications that might not have been retrieved from the database search. Duplicated data and review articles without original series were excluded. When the results of a single study were reported in more than one publication, only the most recent and complete data were included. Table 1 summarises the search strategy.
Table 1
| Items | Specification |
|---|---|
| Date of search | December 1, 2021 |
| Databases and other sources searched | PubMed, Web of Science and Cochrane databases |
| Search terms used | “Laparoscopic”, “Hepatectomy”, “Liver resection”, “Caudate lobe”, “Segment1” |
| Timeframe | From 1st January 2006 until 31st November 2021 |
| Inclusion and exclusion criteria | Inclusion criteria: |
| English-language article; | |
| Article types were case-report, case-series and any study including original series | |
| Exclusion criteria: | |
| Duplicated data; | |
| Non-English language articles; | |
| Editorial or review articles without original series; | |
| Articles including extended procedures other than caudate lobe resection | |
| Selection process | Two independent authors searched for papers |
Results
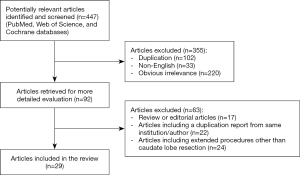
Four hundred forty-seven articles were identified, duplicates (n=102), articles not published in English (n=33), and those of apparent irrelevance (n=220) were excluded, leaving 92 articles. Out of these 92, we excluded review and editorials articles (n=17), articles including a duplication report from the same institution or author (n=22), and articles including extended procedures, other than resection in the posterosuperior segments (n=24). Thus, we finally selected 29 articles about laparoscopic caudate lobe resection. Figure 1 shows the flowchart of the literature search.
Concepts of embryology
In order to understand the surgical anatomy of the liver, we should consider that the caudate lobe and the retro-hepatic vena cava develop as a joint entity that is separate from the remaining liver. The dorsal vein, from which the future caudate develops, becomes the portion of inferior vena cava (IVC) between the renal veins and joins the hepato-cardiac channel containing the supra-hepatic veins. The hepatic venous branches to the caudate drain directly into the vena cava, and a few short branches connect the caudate to the main hepatic veins.
Consequently, segment I should be considered a “peculiar entity” that is embryologically separate from the rest of the liver (13). This intimate connection with the IVC and other important vascular structures, such as the portal bifurcation and the hepatic veins, can explain why the laparoscopic approach to the caudate lobe is still not widely adopted.
History, surgical anatomy and the myth of segment IX
In 1966, Couinaud defined the modern segmental anatomy of the liver, with the Spiegel lobe designated as an independent hepatic segment, named as the dorsal sector, caudate lobe, or Couinaud segment I (14). The caudate lobe lies below the hepatic hilum, very close to the ductal and portal bifurcation, and the hepatic arteries above the inferior vena cava.
According to the most relevant studies published from 1953 to 2000, the caudate lobe can be divided into three main parts or subsegments (Table 2). Kumon’s description, which includes the left or Spiegel lobe, the medial pericaval portion, and the right portion or caudate process, is the most adopted terminology.
Table 2
| Author/year | Caudate lobe subsegments | |||
|---|---|---|---|---|
| Right | Medial | Left | ||
| Healey and Schroy, 1953 (15) | Segment Ir | Segment Ir | Segment Il | 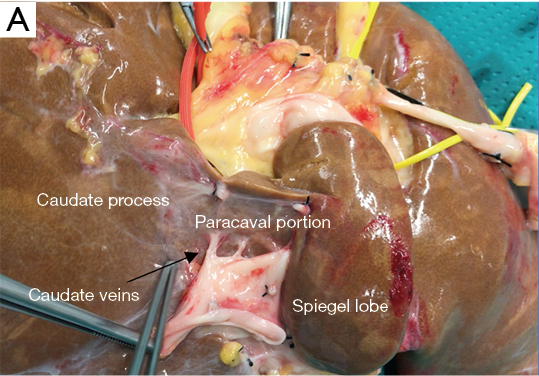 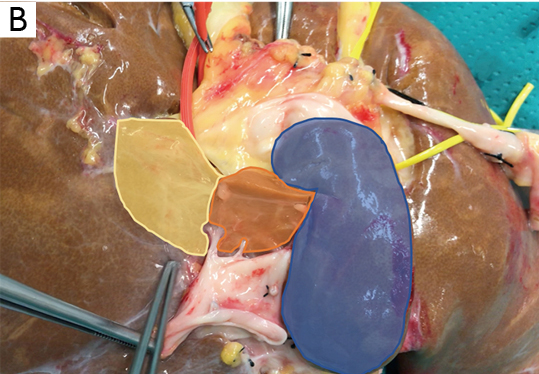 |
| Couinaud, 1981 (16) | Caudate lobe, segment I | Caudate lobe, segment I | Caudate lobe, segment I | |
| Kumon et al., 1985 (17) | Caudate process | Paracaval portion | Spiegel lobe | |
| Elias et al., 1992 (18) | Caudate process | Central part | Left part, spiegel lobe | |
| Couinaud, 1994 (19) | Segment IX b/c | Segment IX d | Segment I | |
| Couinaud, 1999 (20) | Subsegment RDSb | Subsegment RDSb | Left dorsal sector | |
| Filipponi et al., 2000 (21) | Segment IX right | Segment IX left | Segment I | |
| Kogure et al., 2000 (22) | Caudate process | Paracaval portion | Spiegel lobe, papillary process | |
RDS, right dorsal sector.
The first and more easily visible portion of the segment I at laparoscopic exploration is the proper Spiegel lobe, which can be seen to the left of the IVC through the hepato-gastric ligament, protruding from Arantius sulcus, also called the papillary process by Kogure (23).
The birth of the “segment IX” myth is because Couinaud and Filliponi proposed that the part of the caudate lobe to the right of the middle hepatic vein might be a separate segment: segment IX (19,21). However, further analysis of liver casts showed that the proposed border between pericaval portion of segment I and the postulated segment IX is crossed by overlapping portal pedicles. Given that any portal pedicle should not cross a portal fissure, the same authors propose that the entire dorsal liver be considered a single portal segment with three subsegments: the Spiegel lobe pericaval portion and caudate process. So, we believe that the existence of the so-called segment IX is just a myth.
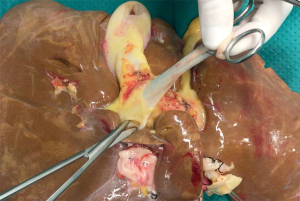
An average of three (one to six) portal branches supplies the entire caudate and may arise from the left, right, bifurcation, or a combination of the portal branches. For example, a portal branch feeding the paracaval portion of the caudate lobe is shown in Figure 2.
The caudate lobe arteries are multiple small branches arising both from the left and right hepatic artery in about half of individuals, whereas in the rest of the patients, a dominant vascularization from left or right can be observed. Venous drainage of the segment I is provided by one or two thick veins (2 to 3 mm in diameter), usually called the “caudate veins” (Table 2, Image A) and several thin veins. The thicker veins enter the IVC, the thinner drain into the IVC or the middle and/or right hepatic vein. This network of hepatic veins forms an anastomotic arcade between the main hepatic veins and vena cava, thus providing venous drainage of the dorsal liver even in case of pathologic thrombosis, also called Budd-Chiari Syndrome. Approximately half of the 2 to 4 ducts from the CL join the right posterior sectorial originating from S6 and S7. The other ducts drain into the left hepatic duct (24).
Caudate lobe resection in consensus conferences, guidelines and difficulty score systems
First Consensus Conference on LLS (Louisville, USA, 2008) stated that the most favourable indications for the laparoscopic resection were a solitary lesion, 5 cm or less, located in peripheral liver segments 2 to 6. In contrast, lesions located in posterior segments (1, 7, and 8) were not universally accepted as the standard of care (1). No specific recommendations about LCL are included in the Second International Consensus Conference (Morioka, Japan, 2014). Nonetheless, it was agreed that the difficulty of the LLR is determined, among others, by the “tumour location and the proximity to major vessels” (2). During the same Conference, the IWATE difficulty scoring system, which is further discussed, was developed.
The European Guidelines Meeting on Laparoscopic Liver Surgery (Southampton, UK, 2017) included segment I among the so-called ‘‘difficult segments” (1, 4a, 7, and 8). The expert panel acknowledged that resections of these segments, especially when anatomical, are “highly complex and require advanced expertise in LLR (25). A new definition of “technically major resection” was applied to LCLs by Halls in 2018. He stated that, even though posterosuperior segments resections would be considered minor (involving only 1 or 2 Couinaud segments), they involve areas of the liver with difficult laparoscopic access (26).
Surprisingly, a formal LCL was not even included in Ban’s novel difficulty scoring system for LLR published in 2014 (27). All the same, the author attributed an additional score of 1 to lesions located in the proximity of major vessels (primary or second branches of Glisson’s tree, major hepatic veins, and IVC). The original Ban’s difficulty scoring system, lacking segment 1 in the tumour location category, had other limitations, such as no separation between segments 4a and 4b and no category for hand-assisted laparoscopic surgery. Because of these limitations, this score was then revised at the Morioka Consensus Conference and renamed the IWATE criteria (an index-based, 4-level classification system) incorporating the difficulty of Segment 1 resection (score 4) (28). Lately, in 2018, Kawaguchi proposed a new difficulty classification of LLR. In this paper, the LCL is included, among other procedures, on posterosuperior segments, in group III, representing the highly advanced level of difficulty.
Laparoscopic approach to caudate lobe resection
LCL has been described in multicentre cohort studies, case series, and case reports, and our review reports a total of 265 patients (Table 3).
Table 3
| Ref. | Number of patients | Indications | Subsegment of caudate lobe resected | Median lesion size (mm) | Blood loss (mL) | Median operation time (min) | Morbidity rate | Complications (general and liver specific) | Laparotomy conversion | Days of hospitalization | Oncological outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Araki et al. (6) | 15 | HCC, metastases, benign | CP, SL, PC | 19.5 | 75 | 150 | 6.6% | BS, bleeding (massive from major vessels) | 0 | 8 | R0 |
| Dulucq et al. (7) | 2 | 2 CRLM | – | – | 150 | 127 | – | – | 0 | 9 | R0 |
| Koffron et al. (8) | 7 | HCC, benign | – | – | – | – | – | – | 0 | – | – |
| Chen et al. (9) | 8 | 4 HCC, 4 metastases | CP, SL, PC | – | 110 | 248 | – | – | 0 | 6.9 | R0 |
| Lai and Tang (10) | 2 | 2 HCC | – | 15 | 143 | 143 | – | – | 0 | 4.5 | R0 |
| Salloum et al. (11) | 5 | 4 HCC, 1 benign | CP, SL, PC | 35 | 200 | 240 | – | – | 0 | 6 | R0 |
| Oh et al. (12) | 6 | 4 HCC, 1 CC, 1 metastases | CP, SL, PC | 26.5 | 242.5 | 382 | – | – | 0 | 8 | R0 |
| Cappelle et al. (29) | 32 | 22 CRLM, 1 CC, 4 unspecified malign lesion, 5 benign | SL, PC, SL | 22 | 100 | 155 | 6.3% | 1 bleeding, 1 LI | 9.4% | 3 | 66.6% (R0); 33.3% (R1) |
| Chai et al. (30) | 6 | CC, HCC | – | 54 | 260 | 249 | – | 1 surgical site infection, 1 pleural effusion | 0 | 7 | – |
| Hayami et al. (31) | 6 | HCC, CRCM | – | 18.3 | 35 | 207 | – | – | 0 | 8 | R0 |
| Ho et al. (32) | 1 | HCC | – | 16 | 200 | 270 | – | – | 0 | 4 | R0 |
| Jin et al. (33) | 12 | CH, HCC, FNH | CP | 55 | 97 | 140 | – | – | 0 | 9.2 | – |
| Koh et al. (34) | 6 | HCC, CRLM | – | 30 | 217 | 318 | – | – | 0 | 4 | R0 |
| Li et al. (35) | 11 | MT, HCC, FNH | – | 33 | 133 | 226 | – | – | 0 | 6 | R0 |
| Liu et al. (36) | 1 | HCC | PC | 25 | – | 300 | – | – | 0 | – | – |
| Machado et al. (37) | 1 | CRLM | – | – | <50 | 120 | – | – | 0 | 3 | R0 |
| Siming et al. (38) | 9 | HL | – | – | 530 | 310 | – | 1 bleeding | 0 | 10 | – |
| Vega et al. (39) | 1 | HCC | SL | 36 | – | – | – | – | 0 | – | R0 |
| Wan et al. (40) | 1 | CC | – | 42 | 180 | 300 | – | – | 0 | 7 | R0 |
| Xiang et al. (41) | 1 | HCC | SL | – | <50 | 196 | – | – | 0 | 6 | – |
| Xu et al. (42) | 19 | CH, FNH, AD, HCC, CRLM | CL, SL, PC | 11.9 | 75 | 186.5 | – | – | 0 | 6 | R0 |
| Ding et al. (43) | 10 | 5 HCC, 5 benign | 10 CL | 60 | 50 | 216 | – | – | – | 15 | – |
| Peng et al. (44) | 31 | 10 HCC, 4 CRLM, 17 benign | 5 SL, 1 SL + PC, 4 PL + PC, 1 SL + PL + PC, 20 tumorectomy | 40 | 100 | 210 | 5 16.1% | 1 infectious diarrhoea, 2 LI, 2 ascites | 3.2% | 5 | – |
| Parikh et al. (45) | 12 | 11 HCC, 1 CRLM | CL | 20 | 250 | 204.5 | 16.7% | 1 bleeding, 1 pleural effusion | 0 | 4 | R0 |
| Ruzzenente et al. (46) | 42 | 11 CRLM, 16 HCC, 3 NCRLM, 12 benign | CL, SL, PC | 35.7 | 173 | 298 | 14.28% | 1 abdominal collection, 1 cardiological, 1 pleural effusion, 3 unknown | 7.1% | 4.7 | 13.3% (R1) |
| Sun et al. (47) | 15 | 5 hemangioma, 4 HCC, 4 FNH, 1 hepatolithiasis, 1 angiomyolipoma | CL, SL, PC | 56 | 400 | 305 | 40% | – | 0 | 9 | R0 |
| Furukawa et al. (48) | 1 | CC | CL | 10 | 400 | 264 | – | – | 0 | 9 | – |
| Yasuda et al. (49) | 1 | HCC | CL | 11 | 10 | 229 | – | – | 0 | 8 | R0 |
| Tur-Martínez et al. (50) | 1 | Hepatocellular adenoma | CL | 18 | 100 | 120 | – | – | 0 | 3 | R0 |
iLCL, isolated laparoscopic caudate lobectomy; HCC, hepatocellular carcinoma; CC, cholangiocarcinoma; BD, benign disease; CRCM, colo-rectal cancer metastases; CH, cavernous hemangioma; FNH, focal nodular hyperplasia; MT, mesenchymal tumours; HL, hepatolithiasis; AD, adenoma; CP, caudate process; SL, Spiegel’s lobe; PC, pericaval portion; BL, biliary leakage; BS, biliary stenosis; BL, bleeding; LI, liver insufficiency.
Laparoscopic resections of lesions located on the segment I were shown to be feasible but remain technically demanding procedures that should be performed by experienced laparoscopic hepatic surgeons.
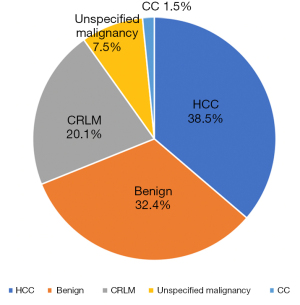
The main indication for CLC included in our review was HCC (38.5%), followed by benign diseases such as hepatolithiasis, focal nodular hyperplasia (FNH) or adenomas (32.4%); or malignancies like colorectal liver metastasis (CRLM) (20.1%), unspecified tumours (7.5%) and cholangiocarcinoma (1.5%) (Figure 3).
It is well recognized that tumor size is a risk factor for conversion and blood loss in LLR. The 2008 Louisville statement recommended that tumours larger than 5 cm in diameter were not candidates for LLR (51). Several reports provided data on the laparoscopic approach for more extensive lesions in recent years despite this recommendation. There is no agreement in the literature on the maximal tumour dimension suitable for laparoscopic caudate resection. In patients considered in our review, the median size of lesions ranged between 12 and 60 mm. Moreover, the possibility of invasion of important structures should be considered in Segment I, especially regarding the paracaval portion (46).
No limitations have been described according to the location of the lesions inside the caudate lobe (caudate process, Spiegel’s lobe, and paracaval portion). Usually, partial resections are well accepted for benign lesions, or CRLM, while for malignancies such as HCC or cholangiocarcinoma, a proper anatomical caudate lobectomy is advocated.
The laparoscopic approach combined with a reverse Trendelenburg position offers a “caudal access” that could provide a better view of liver parenchyma in a narrow surgical field.
As stated by Cappelle et al., LCL is safe and feasible when performed in high-volume centres. However, profound anatomical knowledge, advanced laparoscopic skills, and mastering intraoperative ultrasound are essential (29).
A wide range in operative time and blood loss has been observed among all studies taken into consideration, more likely relating to centre and surgeon experience. No outlying value has been observed, and all of them can be considered belonging to an acceptable range of values for hepatic resections.
Conversion to laparotomy has been reported in seven patients, representing 2.6% of the patients included in this review. One conversion was performed because of difficult localization and enhanced bleeding risk, three for technical difficulties, three for active bleeding (one from the IVC).
The oncological radicality achievement was reported in twenty studies, and positive resection margins were observed in 14 patients (7.7%). For what concerns oncological radicality, as considered in the multicentre retrospective study by Cappelle et al., a negative surgical margin, i.e., 1 mm, is still considered the standard (29). However, in high-volume hepatobiliary units, the occurrence of R1 resection in open caudate lobe resection is reported up to 25% (15). Further studies with long-term follow-up are needed to evaluate differences between the open and laparoscopic approaches in terms of negative margin, disease-free survival, and survival rate.
The morbidity rate reported in the studies included in our review was 6.8% (18/265), and no mortality was recorded. Complications reported were: four bleedings, three liver insufficiencies, two ascites, three pleural effusion, one biliary stenosis, one abdominal collection, one surgical site infection, one cardiological complication, one infective diarrhoea and three unknowns. The results are comparable to the complication rate that occurred in open procedures, according to a metanalysis by Ding et al. (22).
The median length of hospital stay of patients included in this systematic review was six days, ranging between 3 and 15 days of hospitalization, showing a postoperative recovery in line with other laparoscopic hepatic procedures and shorter than the open approach (52).
Operative techniques and features
LCL operative techniques and features have been described in multicentre cohort studies, case series, and case reports (Table 4). All the analysed cases have been carried out laparoscopically. The most used position of the patient was supine with reverse Trendelenburg with an inclination ranging from 15% to 30%. The exceptions were represented by Ho et al., who suggest a lithotomic position for the patient, and Cappelle et al., who reported using left lateral position in 2 cases in their multicentre study (29,32). The number of trocars needed ranged between four and six, with the first one placed periumbilically in most cases and still not a standardized position of accessory trocars still placed according to surgeon’s preference.
Table 4
| Ref. | Number of patients | Operative setting | Patient position | Trocars | CVP intraoperative management | Pringle manoeuvre | Energy devices |
|---|---|---|---|---|---|---|---|
| Araki et al. (6) | 15 | Lps | – | – | – | – | Harmonic and Thunderbeat |
| Dulucq et al. (7) | 2 | Lps | Supine with split legs | 6 trocars | Yes (5 mmHg) | Yes | Ligasure and DISSECTRON |
| Koffron et al. (8) | 7 | Lps | Supine | – | Yes | – | Ligasure |
| Chen et al. (9) | 8 | Lps | Modified lithotomy position | 4 or 5 trocars | – | – | Harmonic |
| Lai and Tang (10) | 2 | Lps (robot-assisted) | Supine with split legs and reverse Trendelenburg | 5 trocars | Yes (<5 mmHg) | – | – |
| Salloum et al. (11) | 5 | Lps | Supine with split legs and reverse Trendelenburg | 6 trocars | Yes (<5 mmHg) | Yes | Ligasure and DISSECTRON |
| Oh et al. (12) | 6 | Lps | Modified lithotomy position | 5 trocars | – | – | – |
| Cappelle et al. (29) | 32 | Lps | Supine in 30° reverse Trendelenburg | 4 or 5 trocars | Yes | Yes | ENSEAL and CUSA |
| Chai et al. (30) | 6 | Lps | Supine with closed legs | 5 trocars | – | Yes | – |
| Hayami et al. (31) | 6 | Lps | Supine with open legs in 15° reverse Trendelenburg | 5 trocars | – | Yes | Harmonic ACE and CUSA |
| Ho et al. (32) | 1 | Lps | Lithotomy position | 5 trocars | – | – | Harmonic, Ligasure and CUSA |
| Jin et al. (33) | 12 | Lps | Supine in 30° reverse | 6 trocars | – | Yes | – |
| Koh et al. (34) | 6 | Lps | Supine with split legs | 5 trocars | Yes (5 mmHg) | Yes | CUSA |
| Li et al. (35) | 11 | Lps | Supine | 5 trocars | Yes (<5 mmHg) | Yes | Harmonic |
| Liu et al. (36) | 1 | Lps | Supine | – | – | Yes | Harmonic, Ligasure and CUSA |
| Machado et al. (37) | 1 | Lps | – | 4 trocars | – | – | Bipolar |
| Siming et al. (38) | 9 | Lps | Supine with split legs | 5 trocars | – | – | – |
| Vega et al. (39) | 1 | Lps | Supine with split legs | – | Yes | – | – |
| Wan et al. (40) | 1 | Lps | Supine with split legs | 5 trocars | Yes (<5 mmHg) | Yes | Harmonic, ACE and CUSA |
| Xiang et al. (41) | 1 | Lps | Supine with split legs | 5 trocars | Yes | – | – |
| Xu et al. (42) | 19 | Lps | Supine with split legs | 5 trocars | Yes (<5 mmHg) | Yes | CUSA |
| Ding et al. (43) | 10 | Lps | Supine with split legs | 5 trocars | Yes (between 4–6 mmHg) | Yes | Ultrasonic scalpel |
| Peng et al. (44) | 31 | Lps | Supine | 5 trocars | Yes (<5 mmHg) | Yes | Harmonic, Ligasure and CUSA |
| Parikh et al. (45) | 12 | Lps | Supine | 5 trocars | – | – | Harmonic, Ligasure and CUSA |
| Ruzzenente et al. (46) | 42 | Lps | Supine | 4 trocars | – | Yes | – |
| Sun et al. (47) | 15 | Lps | Supine with split legs | 5 trocars | Yes (between 3–5 mmHg) | Yes | Ultrasonic scalpel |
| Furukawa et al. (48) | 1 | Lps | Supine | – | – | – | Ultrasonic scalpel |
| Yasuda et al. (49) | 1 | Lps | Supine | – | – | Yes | Harmonic, CUSA |
| Tur-Martínez et al. (50) | 1 | Lps | Supine with split legs | 5 trocars | – | Yes | Ligasure |
iLCL, isolated laparoscopic caudate lobectomy; CVP, central venous pressure; Lps, laparoscopic; CUSA, cavitron ultrasonic surgical aspirator.
Pringle manoeuvre has been applied for better bleeding control during transection of the hepatic parenchyma in most centres (19/31). Notably, encircling the hepatic pedicle also allows better exposition of the operative field by retracting the hepatic hilum to the right or left side depending on the caudate lobe portion to be addressed.
Both left or right approaches are described sharing the same operative steps that can be summarized in caudate lobe exposure; inflow control by clipping/ligating and dividing the portal triad (Arantius plate dissection is crucial on the left side); outflow control by clipping/ligating and dividing the caudate veins draining into IVC (this goal is obtained lifting the caudate lobe and exploiting the laparoscopic caudal view); parenchymal transection. The intra-Glissonean approach to caudate lobe pedicles was the most frequently adopted in almost all the studies included in this review. However, we found a couple of reports describing the extrahepatic Glissonean approach in laparoscopic caudate lobe resection. Cai et al. (30) dissected and divided caudate portal triads one at a time in their series of 11 cases. In contrast, a laparoscopic anatomic Spiegel lobectomy using a detailed extrahepatic Glissonean approach (with an edited video) has been reported by Xiang et al. (41).
From the technical point of view, it is still controversial whether to isolate and control the main vascular structures (as the left suprahepatic vein) extrahepatically.
Laparoscopic selective clamping of blood supply for visualization of ischemia demarcation line can also be used in anatomical resection of segment I (51).
IOUS has been used for lesion localization, outline major vessel proximity, and resection guidance in almost all reviewed cases.
Surgical instruments for laparoscopic surgery have advanced remarkably in recent years, resulting in safer procedures for LLR, even for resection of lesions in difficult segments.
Innovative technologies applied to resections of difficult hepatic segments include pre-operative 3-D CT-scan reconstructions, ICG green demarcation, and the use of 3-D laparoscopic equipment. In addition, real-time virtual sonography during liver surgery has been reported recently. This new imaging technology detects the spatial position of an ultrasound probe and reconstructs a section of computed tomography and/or magnetic resonance images in accordance with the ultrasound image (52).
The energy devices used in all series included ultrasonic dissector, radiofrequency, bipolar energy. All used advanced dissectors are listed in Table 3.
Not everyone opted for intraoperative central venous pressure (CVP) monitoring. Nonetheless, many reports have shown that reduction of cardiac preload leads to a decrease in hepatic vein congestion and reduction of intraoperative blood loss (52-54). Historically central venous pressure was used to evaluate cardiac preload (54). More recently, in the era of mini-invasive surgery, stroke volume variation has been reported as a non-invasive approach to guide fluid management. This approach improves intraoperative outcomes in laparoscopic liver surgery, thus enhancing the benefits of the minimally-invasive approach and fast-track protocols (55).
The site of specimen extraction was either through a trocar enlargement, previous incision, and Pfannenstiel incision. The majority of studies reported the use of one of the previous incisions without specifying the site of extraction used and according to the patient’s characteristics and surgeon attitude.
Final considerations
Patients presenting primary hepatic tumours or metastases in the caudate lobe can easily lose the possibility of radical oncological resection because of the easy and early possibility of the inferior vena cava infiltration. Thus, it is crucial to offer them a laparoscopic approach as one of the possible operative techniques. LCL requires a deep anatomic knowledge of the caudate lobe and advanced technical expertise.
As stated by Xu et al., for caudate lobectomy, the laparoscopic technique is a double-edged sword (42). On the one hand, LCL resection can minimize blood loss, decrease post-operative pain and reduce the length of the hospital stay, but on the other hand, the patient can face the risk of massive intra-operative bleeding and a positive tumour margin. As a result, strict patient selection and experienced surgeons who specialize in both open liver surgery and laparoscopic surgery are needed during this procedure.
A multicenter, propensity score-matched analysis of safety, feasibility, and early outcomes in LCL resection performed in eighteen patients reported that the laparoscopic approach is a feasible choice for resection of lesions located in segment one with acceptable outcome (42).
A multi-institutional propensity score-matched cohort study evaluated the safety and feasibility of LCL in the Italian prospective maintained database on minimally invasive liver surgery (IgoMILS) by comparison with a cohort of patients submitted to open CL resections. LCL compared to open CL had lower intraoperative blood loss, reduced use of abdominal drainage and lower comprehensive complication index (CCI) (45).
Chai et al. affirmed that compared with open surgery, the laparoscopic approach could offer a unique viewing angle from below, and the superior magnification and illumination could further increase the visibility of this visually restricted area, which make isolated LCL an ideal oncological resection for tumours confined to the caudate lobe (30).
A recent meta-analysis comparing open and laparoscopic caudate resection found better postoperative outcomes in terms of hospital stay, intraoperative blood loss, operation time, and intraoperative blood transfusion requirements without an increased morbidity rate (22).
Cost-effectiveness analysis of laparoscopic approach compared to the open for CLC is still lacking.
It can be stated that the laparoscopic approach to the caudate lobe is not near future anymore but instead represents the current reality when considered by high-expertise surgeons working in high-volume hepatobiliary centres. However, since the lack of high-quality evidence and the scarcity of cases, prospective clinical trials, and multi-centre randomised controlled trials are needed to standardize this technique.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Edoardo Rosso and Santiago Azagra) for the series “Focus on Technical Advancement in Mini-invasive HPB Surgery” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-2/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-2/coif). The series “Focus on Technical Advancement in Mini-invasive HPB Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Skandalakis JE, Skandalakis LJ, Skandalakis PN, et al. Hepatic surgical anatomy. Surg Clin North Am 2004;84:413-35. viii. [Crossref] [PubMed]
- Scuderi V, Barkhatov L, Montalti R, et al. Outcome after laparoscopic and open resections of posterosuperior segments of the liver. Br J Surg 2017;104:751-9. [Crossref] [PubMed]
- Fuji H, Hatano E, Seo S, et al. Prospective registry for laparoscopic liver resection. Asian J Endosc Surg 2017;10:173-8. [Crossref] [PubMed]
- Araki K, Fuks D, Nomi T, et al. Feasibility of laparoscopic liver resection for caudate lobe: technical strategy and comparative analysis with anteroinferior and posterosuperior segments. Surg Endosc 2016;30:4300-6. [Crossref] [PubMed]
- Dulucq JL, Wintringer P, Stabilini C, et al. Isolated laparoscopic resection of the hepatic caudate lobe: surgical technique and a report of 2 cases. Surg Laparosc Endosc Percutan Tech 2006;16:32-5. [Crossref] [PubMed]
- Koffron AJ, Auffenberg G, Kung R, et al. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg 2007;246:385-92; discussion 392-4. [Crossref] [PubMed]
- Chen KH, Jeng KS, Huang SH, et al. Laparoscopic caudate hepatectomy for cancer--an innovative approach to the no-man's land. J Gastrointest Surg 2013;17:522-6. [Crossref] [PubMed]
- Lai EC, Tang CN. Robot-assisted laparoscopic partial caudate lobe resection for hepatocellular carcinoma in cirrhotic liver. Surg Laparosc Endosc Percutan Tech 2014;24:e88-91. [Crossref] [PubMed]
- Salloum C, Lahat E, Lim C, et al. Laparoscopic Isolated Resection of Caudate Lobe (Segment 1): A Safe and Versatile Technique. J Am Coll Surg 2016;222:e61-6. [Crossref] [PubMed]
- Oh D, Kwon CH, Na BG, et al. Surgical Techniques for Totally Laparoscopic Caudate Lobectomy. J Laparoendosc Adv Surg Tech A 2016;26:689-92. [Crossref] [PubMed]
- Lassau JP, Hureau J. Note on the organogenesis of the retro-hepatic segment of the vena cava in humans. France: CR Ass Anat, 1968.
- Couinaud C. Surgical anatomy of the liver revisited. Paris, France: 1966.
- Healey JE Jr, Schroy PC. Anatomy of the biliary ducts within the human liver; analysis of the prevailing pattern of branchings and the major variations of the biliary ducts. AMA Arch Surg 1953;66:599-616. [Crossref] [PubMed]
- Couinaud C. Controlled Hepatectomies and Exposure of the Intrahepatic Bile Ducts: Anatomical and Technical Study. France: 1981.
- Kumon M, Kumon T, Tsutsui E, et al. Definition of the caudate lobe of the liver based on portal segmentation. Glob Health Med 2020;2:328-36. [Crossref] [PubMed]
- Elias D, Lasser PH, Desruennes E, et al. Surgical approach to segment I for malignant tumors of the liver. Surg Gynecol Obstet 1992;175:17-24. [PubMed]
- Couinaud C. Intrahepatic anatomy: application to liver transplantation. Ann Radiol 1994;37:323-33. [PubMed]
- Couinaud C. Secteur dorsal du foie (Dorsal sector of the liver). Chirurgie 1998;123:8-15. [Crossref] [PubMed]
- Filipponi F, Romagnoli P, Mosca F, et al. The dorsal sector of human liver: embryological, anatomical and clinical relevance. Hepatogastroenterology 2000;47:1726-31. [PubMed]
- Ding Z, Liu L, Xu B, et al. Safety and feasibility for laparoscopic versus open caudate lobe resection: a meta-analysis. Langenbecks Arch Surg 2021;406:1307-16. [Crossref] [PubMed]
- Kogure K, Kuwano H, Fujimaki N, et al. Relation among portal segmentation, proper hepatic vein, and external notch of the caudate lobe in the human liver. Ann Surg 2000;231:223-8. [Crossref] [PubMed]
- Abdalla EK, Vauthey JN, Couinaud C. The caudate lobe of the liver: implications of embryology and anatomy for surgery. Surg Oncol Clin N Am 2002;11:835-48. [Crossref] [PubMed]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 2018;268:11-8. [Crossref] [PubMed]
- Halls MC, Berardi G, Cipriani F, et al. Development and validation of a difficulty score to predict intraoperative complications during laparoscopic liver resection. Br J Surg 2018;105:1182-91. [Crossref] [PubMed]
- Ban D, Tanabe M, Ito H, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci 2014;21:745-53. [Crossref] [PubMed]
- Tanaka S, Kawaguchi Y, Kubo S, et al. Validation of index-based IWATE criteria as an improved difficulty scoring system for laparoscopic liver resection. Surgery 2019;165:731-40. [Crossref] [PubMed]
- Cappelle M, Aghayan DL, van der Poel MJ, et al. A multicenter cohort analysis of laparoscopic hepatic caudate lobe resection. Langenbecks Arch Surg 2020;405:181-9. [Crossref] [PubMed]
- Chai S, Zhao J, Zhang Y, et al. Arantius Ligament Suspension: A Novel Technique for Retraction of the Left Lateral Lobe Liver During Laparoscopic Isolated Caudate Lobectomy. J Laparoendosc Adv Surg Tech A 2018;28:740-4. [Crossref] [PubMed]
- Hayami S, Ueno M, Kawai M, et al. Standardization of surgical procedures for laparoscopic Spiegel lobectomy: A single-institutional experience. Asian J Endosc Surg 2019;12:232-6. [Crossref] [PubMed]
- Ho KM, Han HS, Yoon YS, et al. Laparoscopic Total Caudate Lobectomy for Hepatocellular Carcinoma. J Laparoendosc Adv Surg Tech A 2017;27:1074-8. [Crossref] [PubMed]
- Jin B, Jiang Z, Hu S, et al. Surgical Technique and Clinical Analysis of Twelve Cases of Isolated Laparoscopic Resection of the Hepatic Caudate Lobe. Biomed Res Int 2018;2018:5848309. [Crossref] [PubMed]
- Koh YX, Lee SY, Chiow AKH, et al. Laparoscopic caudate lobe resection: navigating the technical challenge. Ann Laparosc Endosc Surg 2017;2: [Crossref]
- Li Y, Zeng KN, Ruan DY, et al. Feasibility of laparoscopic isolated caudate lobe resection for rare hepatic mesenchymal neoplasms. World J Clin Cases 2019;7:3194-201. [Crossref] [PubMed]
- Liu F, Wei Y, Li B. Laparoscopic Isolated Total Caudate Lobectomy for Hepatocellular Carcinoma Located in the Paracaval Portion of the Cirrhotic Liver. Ann Surg Oncol 2019;26:2980. [Crossref] [PubMed]
- Machado MA, Surjan R, Bassères T, et al. Laparoscopic resection of caudate lobe. Technical strategies for a difficult liver segment – Video article. Surg Oncol 2018;27:674-5. [Crossref] [PubMed]
- Siming Z, Jie Z, Hong L, et al. Laparoscopic caudate lobe resection for the treatment of hepatolithiasis. J Minim Access Surg 2019;16:106-10. [Crossref] [PubMed]
- Vega EA, Nicolaescu DC, Salehi O, et al. Laparoscopic Segment 1 with Partial IVC Resection in Advanced Cirrhosis: How to Do It Safely. Ann Surg Oncol 2020;27:1143-4. [Crossref] [PubMed]
- Wan HF, Xie KL, Li JX, et al. Laparoscopic Caudate Lobectomy for Cholangiocarcinoma of Caudate Lobe Invading Middle Hepatic Vein. Ann Surg Oncol 2020;27:4181-5. [Crossref] [PubMed]
- Xiang S, Zhang YX, Chai SS, et al. Laparoscopic Anatomic Spiegel Lobectomy with the Extrahepatic Glissonean Approach. Surg Laparosc Endosc Percutan Tech 2019;29:e57-9. [Crossref] [PubMed]
- Xu G, Tong J, Ji J, et al. Laparoscopic caudate lobectomy: a multicenter, propensity score-matched report of safety, feasibility, and early outcomes. Surg Endosc 2021;35:1138-47. [Crossref] [PubMed]
- Ding Z, Huang Y, Liu L, et al. Comparative analysis of the safety and feasibility of laparoscopic versus open caudate lobe resection. Langenbecks Arch Surg 2020;405:737-44. [Crossref] [PubMed]
- Peng Y, Liu F, Xu H, et al. Propensity score matching analysis for outcomes of laparoscopic versus open caudate lobectomy. ANZ J Surg 2021;91:E168-73. [Crossref] [PubMed]
- Parikh M, Han HS, Cho JY, et al. Laparoscopic isolated caudate lobe resection. Sci Rep 2021;11:4328. [Crossref] [PubMed]
- Ruzzenente A, Ciangherotti A, Aldrighetti L, et al. Technical feasibility and short-term outcomes of laparoscopic isolated caudate lobe resection: an IgoMILS (Italian Group of Minimally Invasive Liver Surgery) registry-based study. Surg Endosc 2022;36:1490-9. [Crossref] [PubMed]
- Sun TG, Wang XJ, Cao L, et al. Laparoscopic anterior hepatic transection for resecting lesions originating in the paracaval portion of the caudate lobe (with videos). Surg Endosc 2021;35:5352-8. [Crossref] [PubMed]
- Furukawa K, Onda S, Ikegami T. Left Intercostal Approach for Laparoscopic Isolated Total Caudate Lobectomy (with Video). J Gastrointest Surg 2022;26:513. [Crossref] [PubMed]
- Yasuda J, Haruki K, Ikegami T. Laparoscopic Caudate Hepatectomy for a Recurrent Tumor Behind the Vena Cava After Multiple Open Hepatectomies (With Video). J Gastrointest Surg 2021;25:2163-4. [Crossref] [PubMed]
- Tur-Martínez J, Herrero-Fonollosa È, García-Domingo MI, et al. Laparoscopic Caudal Approach of the Inferior Vena Cava for Isolated Segment 1 Liver Resection. J Gastrointest Cancer 2021;52:1180-2. [Crossref] [PubMed]
- Zhuo LW, Prasoon P, Wu H. Role of vascular clamping in hepatic resection: a review. Hepatogastroenterology 2014;61:385-7. [PubMed]
- Satou S, Aoki T, Kaneko J, et al. Initial experience of intraoperative three-dimensional navigation for liver resection using real-time virtual sonography. Surgery 2014;155:255-62. [Crossref] [PubMed]
- Smyrniotis V, Kostopanagiotou G, Theodoraki K, et al. The role of central venous pressure and type pf vascular control inblood loss during major hepatic resections. Am J Surg 2004;187:398-402. [Crossref] [PubMed]
- Melendez JA, Arslan V, Fischer ME, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg 1998;187:620-5. [Crossref] [PubMed]
- Ratti F, Cipriani F, Reineke R, et al. Intraoperative monitoring of stroke volume variation versus central venous pressure in laparoscopic liver surgery: a randomized prospective comparative trial. HPB (Oxford) 2016;18:136-44. [Crossref] [PubMed]
Cite this article as: Anselmo A, Siragusa L, Vinci D, Sensi B, Cascone C, Bacchiocchi G, Vita G, Pellicciaro M, Podda M, Ielpo B, Tisone G. Knowledge and surgical strategies for successful laparoscopic caudate lobectomy: a narrative review. Dig Med Res 2022;5:25.