Diagnostic conundrum of hepatobiliary ascariasis: catch me if you can—case report and literature review
Introduction
Worldwide, ascariasis caused by the nematode Ascaris lumbricoides has led to more than a billion infections (1). It is found predominantly in the tropics and subtropics, where heavy rainfall occurs. The increased soil moisture and humid weather are favourable conditions that encourage worm propagation. In addition, issues like overcrowding, poor sanitation and unhygienic practices make human infection and subsequent cross-transmission a perennial problem in this geographical region (2).
Hepatobiliary ascariasis is a well-described clinical entity of the nematode infection that is common in young adults. Most infected patients remain asymptomatic and hence undiagnosed. However, the rest may present with symptoms of biliary colic, acalculous cholecystitis, ascending cholangitis, acute pancreatitis, recurrent pyogenic cholangitis, and liver abscess (3). In the past, hepatobiliary ascariasis was often discovered late and only during surgery or autopsy, owing to the lack of non-invasive assessment tools. Lately, the wide availability and adoption of ultrasound and endoscopic assessment have increased awareness of this clinical entity (4). Yet, despite improvements, the ability to identify and manage hepatobiliary ascariasis remains a diagnostic challenge. The elusive nature of the mobile worms and the lack of symptoms make eradication difficult and impractical.
Our case demonstrates the chanced discovery of a live worm in a young, overweight lady with no antecedent medical or surgical issues presenting with acute cholangitis. Preliminary investigations were indicative of choledocholithiasis, and there was no need for consideration for other competing etiology. In hindsight, we were fortunate to catch a glimpse of the worms at the exact opportune moment, which helped direct our diagnostic pathway and subsequent management efforts. We present the following case in accordance with the CARE reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-10/rc).
Case presentation
A 36-year-old lady presented with a three-day history of high-grade fever, vomiting, right hypochondrial pain and jaundice in keeping with ascending cholangitis. Physical examination revealed her blood pressure to be 86/51 mmHg (on inotropic support), pulse rate of 163 beats per minute, respiratory rate of 28 breaths per minute and temperature of 40.2 ℃. She was clinically icteric with tenderness over the right hypochondrium. There was no organomegaly, and other system examinations were unremarkable. Relevant blood investigations revealed significantly raised total white blood count of 69.50×103/µL [normal range, (4.00–10.00)×103/µL], deranged liver function test with alanine transaminase of 978 U/L (normal range, 0–55 U/L), aspartate transaminase of 921 U/L (normal range, 5–34 U/L), alkaline phosphatase of 692 U/L (normal range, 40–150 U/L) and total bilirubin of 358.8 µmol/L (normal range, 3.4–20.5 µmol/L) with direct bilirubin predominance of 256.0 µmol/L (normal range, 0.0–8.6 µmol/L). The marked leucocytosis was reactive due to ongoing severe infection, confirmed on full blood picture. Other notable results included a low albumin of 24 g/L (normal range, 35–50 g/L), raised C-reactive protein of 323.8 mg/L (normal range, less than 5.0 mg/L), elevated lactate at 4.86 mmol/L (normal range, 0.50–2.20 mmol/L), deranged renal function with creatinine of 204.2 µmol/L (normal range, 50.4–98.1 µmol/L) and severe metabolic acidosis. The overall clinical assessment and biochemical results pointed towards severe septic shock secondary to ascending cholangitis. Blood cultures were, however, unremarkable. Subsequent hepatobiliary ultrasound performed confirmed the presence of a small, rounded 10 mm calculus impacted within the distal common bile duct (CBD) with resultant upstream biliary dilatation. In addition to this, there were also multiple gallbladder stones of varying sizes. The etiology of cholangitis at this point was secondary to choledocholithiasis.
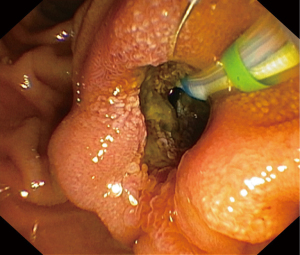
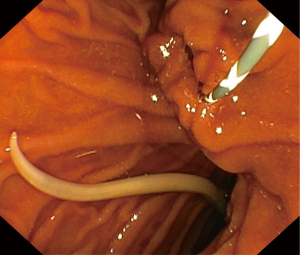
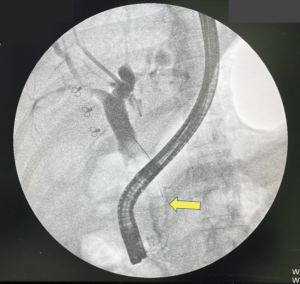
Following resuscitative measures, an endoscopic retrograde cholangiopancreatography (ERCP) revealed an inflamed and patulous ampullary orifice discharging purulent bile (Figure 1). Just distal to this, a live worm was found within the duodenal lumen (Figure 2). Minimal cholangiography performed in the interest of anatomical delineation led to the discovery of a linear, tubular filling defect in the distal CBD (Figure 3). We did not proceed with balloon trawling to reveal the diagnosis as the patient was profoundly septic; hence we had to shorten the procedural time in her best interest. Despite this, the collective findings on ultrasound and ERCP were strongly indicative of a mixed choledocholithiasis and hepatobiliary ascariasis picture. Following stent placement, she improved with wide-spectrum antibiotics and a 5-day course of oral Albendazole.
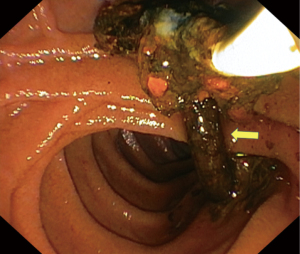
Repeated ERCP three months later with balloon trawling yielded calcified and encrusted tubular structure reminiscent of dead worms (Figure 4). Her condition remained stable on subsequent clinic follow-ups, and she was discharged back to primary care.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The issue with hepatobiliary ascariasis lies in the challenge of catching the worms ‘red-handed’ within the biliary ducts. This is owing to the frequent ease of worm movement in and out of the biliary tree which in its absence, markedly affects the sensitivity (25–91%) of ultrasound confirmation (5). The classical long, tubular filling defect with writhing movements is a major diagnostic give-away on ultrasound but this depends on the exact whereabouts of the worms during the timing of assessment (6).
Physicians may rely on several supportive clinical indicators for hepatobiliary ascariasis to guide diagnosis. These include a background understanding of geohelminth endemicity, history of cholecystectomy for gallstone disorders, and recurrent hepatobiliary symptoms. These clues, however, are not sensitive in ruling out other coexistent hepatobiliary disorders such as stones, malignancy, choledochal cyst, and stricturing disorders (7). Nevertheless, a stepwise investigation is pragmatic in narrowing down the list of these differentials and pointing clinicians towards the correct path. The only clue that is relevant to our patient’s clinical presentation is her hometown of origin, known for its endemicity for ascariasis. Alone, this makes it clinically challenging to diagnose hepatobiliary ascariasis as a potential etiology. Furthermore, the presence of a gallstone within the CBD detected on ultrasound was more than sufficient in establishing a noteworthy etiology that prompts subsequent endoscopic therapy.
Globally, gallstone pathology and choledocholithiasis comprised the majority of all benign hepatobiliary pathologies (8). In most instances, diagnosis is often straightforward following clinical assessment and confirmation on radiological investigations. The challenge arises when hepatobiliary ascariasis coexists with choledocholithiasis like in our case. The frequent migratory behavior of the worms and difficulty in locating them on radioimaging may lead to false reassurances following complete clearance of gallstones during ERCP and subsequent cholecystectomy. As a result, this may undermine the rightful source of cholangitis and lead to recurrent episodes of similar presenting symptoms that continue to mystify the clinician.
In our patient, the discovery of a live worm within the distal duodenum and an inflamed, wide ampulla was indeed fortuitous. There are reported cases with images of the worms migrating across the ampullary orifice, thus providing ample evidence to establish cause (9-11). However, cases where the worms have successfully passed into the duodenum leaving behind a trail of an inflamed ampulla, are not well documented. The patulous ampullary orifice and inflammation are due to worm passage which causes direct mucosal irritation and injury (12). We were only able to establish a probable link from our endoscopic and cholangiographic assessment during the first ERCP. This matter was put to rest when subsequent ERCP with balloon trawling confirmed our diagnosis of hepatobiliary ascariasis. During this later procedure, multiple linear, imbricated structures in keeping with dead worms were trawled out together with stones. The coexisting stones could arise separately from gallstone pathology or stone accretion developing over a nidus from immobile, dead worms (8).
It is mindful to remember that treatment does not conclude with endotherapy and oral vermifuge agents. Health education on personal hygiene and sanitation are salient measures to prevent reinfection. Children living in rural areas are particularly prone to getting infected and remain asymptomatic for a long time. They are potential candidates to develop challenging sequelae from ascariasis in the second and third decades of their lives. It is thus pertinent to educate the affected community on hygienic practices on top of subscribing to regular deworming protocols (13).
Acknowledgments
We wish to express our sincere appreciation by acknowledging our team of gastrointestinal nurses and medical assistants who assisted us and provided valuable insights and feedback during the procedure.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-10/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-10/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Crompton DW. The prevalence of Ascariasis. Parasitol Today 1988;4:162-9. [Crossref] [PubMed]
- Botero D. Epidemiology and public health importance of intestinal nematode infections in Latin America. Prog Drug Res 1975;19:28-43. [Crossref] [PubMed]
- Sanai FM, Al-Karawi MA. Biliary ascariasis: report of a complicated case and literature review. Saudi J Gastroenterol 2007;13:25-32. [Crossref] [PubMed]
- Das AK. Hepatic and biliary ascariasis. J Glob Infect Dis 2014;6:65-72. [Crossref] [PubMed]
- Schulman A. Ultrasound appearances of intra- and extrahepatic biliary ascariasis. Abdom Imaging 1998;23:60-6. [Crossref] [PubMed]
- Larrubia JR, Ladero JM, Mendoza JL, et al. The role of sonography in the early diagnosis of biliopancreatic Ascaris infestation. J Clin Gastroenterol 1996;22:48-50. [Crossref] [PubMed]
- Khuroo MS, Zargar SA. Biliary ascariasis. A common cause of biliary and pancreatic disease in an endemic area. Gastroenterology 1985;88:418-23. [Crossref] [PubMed]
- Khuroo MS, Zargar SA, Yattoo GN, et al. Worm extraction and biliary drainage in hepatobiliary and pancreatic ascariasis. Gastrointest Endosc 1993;39:680-5. [Crossref] [PubMed]
- Ibrarullah M, Mishra T, Dash AP, et al. Biliary ascariasis--role of endoscopic intervention. Trop Gastroenterol 2011;32:210-3. [PubMed]
- Mohd Noor NA, Goh SN, Tan CH. Biliary Ascariasis: An Unusual Case of Obstructive Jaundice. Clin Gastroenterol Hepatol 2020;18:A16. [Crossref] [PubMed]
- Krige J, Shaw J. Cholangitis and pancreatitis caused by biliary ascariasis. Clin Gastroenterol Hepatol 2009;7:A30. [Crossref] [PubMed]
- Sandouk F, Haffar S, Zada MM, et al. Pancreatic-biliary ascariasis: experience of 300 cases. Am J Gastroenterol 1997;92:2264-7. [PubMed]
- Lim-Leroy A, Chua TH. Prevalence and risk factors of geohelminthiasis among the rural village children in Kota Marudu, Sabah, Malaysia. PLoS One 2020;15:e0239680. [Crossref] [PubMed]
Cite this article as: Chiam KH, Muthukaruppan R. Diagnostic conundrum of hepatobiliary ascariasis: catch me if you can—case report and literature review. Dig Med Res 2022;5:38.