A Chinese survey of current practice patterns of preoperative bowel preparation in colorectal surgery
Introduction
Bowel preparation has long been considered as the standard preoperative management for colorectal surgery. A clean colon is thought to facilitate colorectal surgery manipulation, allowing the passage and firing of surgical staplers. Inadequately prepared intestinal tract is considered to be an important factor leading to poor wound healing and postoperative infection, namely surgical site infection (SSI). Among all elective surgeries, colorectal surgery has the highest incidence of SSI, with a recent review showing rates ranging from 5.4% to 23.2% (1).
The method and practice of bowel preparation including mechanical bowel preparation (MBP) and oral antibiotics preparation (OAP) vary differently. MBP refers to the use of oral laxatives and enema to reduce intestinal contents preoperatively, so as to provide a relatively clean intestinal cavity for the surgery, decrease intraluminal bacterial concentration and reduce postoperative infections. OAP refers to the prophylactic use of antibiotics before surgery for the reduction of the bacterial load of the whole body and intestines, so as to reduce the incidence of postoperative infections and other complications.
After the enhanced recovery after surgery (ERAS) concept was proposed, the importance of bowel preparation gradually declined. A multicenter randomized trial of 1,354 patients found that it was reasonable to safely perform colorectal surgery without MBP (2). Some meta-analyses concluded that MBP can be safely omitted in colon surgery (3), even increase the risk of SSI (4). On the other hand, the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) indicated that the combined use of MBP and OAP was associated with a significantly lower incidence of postoperative complications compared with the use of other bowel preparation strategies (5).
The view on whether the preoperative bowel preparation is necessary remains inconclusive, how surgeons manage bowel preparation in the real life clinical practice may be beneficial to the renewal of ideas. The aim of this study is to describe the current attitudes and practice patterns of preoperative bowel preparation among Chinese surgeons. We present the following article in accordance with the SURGE reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-22/rc).
Methods
An online 10-question anonymous survey was announced by posters to the active members who attended the 14th Chinese Academic Congress of Colorectal Surgery (CACCRS 2021) on October 15–17, 2021 (Appendix 1). We distributed the questionnaire by Wenjuanxing web-application (https://www.wjx.cn/). The participants could complete the questionnaire during the meeting. No incentives were offered to obtain the survey results. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of The Seventh Affiliated Hospital of Sun Yat-sen University (No. KY-2020-024-01) and individual consent for this cross-sectional survey was waived. The questionnaire sought information on each surgeon’s current practice of preoperative bowel preparation. In the questionnaire, we collected demographic information, including gender, age, working experience, medical specialty, hospital setting, department volume and monthly number of operations. In terms of bowel preparation, we investigated the reasons for bowel preparation, the methods of preoperative bowel preparation and the choices of agents in the participants’ practice. Among the reasons, we provided options such as preventing SSI, avoiding anastomotic leakage, and reducing risk of postoperative bleeding. Options of bowel preparation methods include using laxatives, enema, OAP and their combinations. We also asked for information on the choices of laxatives, regarding polyethylene glycol-electrolyte lavage solution (PEG-ELS), sodium phosphate (NaP), magnesium sulfate (MgSO4) and mannitol.
We analyzed the data statistically based on the responses obtained. All statistical analyses were performed using STATA version 12.0. Only P value <0.05 was considered statistically significant.
Results
Demographics
A total of 384 Chinese surgeons from 26 provincial administrative regions took part in this survey. The relevant demographic characteristics of the participants are presented in Table 1. Of the 384 participants, 311 (81.0%) were male surgeons. Most of them were between the ages of 40–50 (46.4%), had more than 20 years working experience (62.5%) and worked in general surgery (38.0%). Most of the hospitals were general hospitals (95.3%) and 52.6% department had less than 50 beds. Among the surgeons, 60.9% performed less than 100 operations per month.
Table 1
| Characteristics | Number | Percent (%) |
|---|---|---|
| Gender | ||
| Male | 311 | 81.0 |
| Female | 73 | 19.0 |
| Age | ||
| <40 years | 84 | 21.8 |
| 40–50 years | 178 | 46.4 |
| >50 years | 122 | 31.8 |
| Working experience | ||
| <10 years | 35 | 9.1 |
| 10–20 years | 109 | 28.4 |
| >20 years | 240 | 62.5 |
| Medical specialty | ||
| Gastrointestinal surgery | 110 | 28.7 |
| Anorectal surgery | 128 | 33.3 |
| General surgery | 146 | 38.0 |
| Hospital setting | ||
| General | 366 | 95.3 |
| Specialized | 18 | 4.7 |
| Department volume | ||
| <50 | 202 | 52.6 |
| 50–100 | 142 | 37.0 |
| 101–150 | 22 | 5.7 |
| >150 | 18 | 4.7 |
| Operations per month | ||
| <100 | 234 | 60.9 |
| 100–200 | 122 | 31.8 |
| >200 | 28 | 7.3 |
Bowel preparation reasons, methods and agents
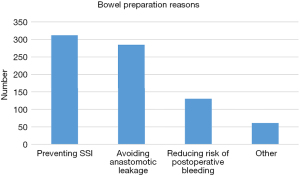
Table 2 shows bowel preparation reasons, methods and agents. The most common reason for choosing bowel preparation was preventing SSI (312/384, 81.3%). Meanwhile, 74.2% Chinese surgeons believed bowel preparation could avoid anastomotic leakage. Only 33.9% thought that bowel preparation was considered to reduce risk of postoperative bleeding (Figure 1).
Table 2
| Answers | Number | Percent (%) |
|---|---|---|
| Reasons (multiple choice) | ||
| Preventing SSI | 312 | 81.3 |
| Avoiding anastomotic leakage | 285 | 74.2 |
| Reducing risk of postoperative bleeding | 130 | 33.9 |
| Other | 61 | 15.9 |
| Methods (single choice) | ||
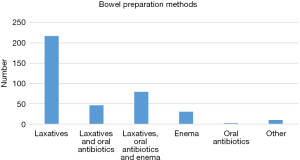
| Laxatives | 217 | 56.5 |
| Laxatives and oral antibiotics | 46 | 12.0 |
| Laxatives, oral antibiotics and enema | 79 | 20.6 |
| Enema | 30 | 7.8 |
| Oral antibiotics | 2 | 0.5 |
| Other | 10 | 2.6 |
| Agents (multiple choice) | ||
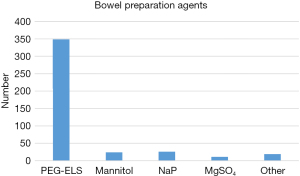
| PEG-ELS | 349 | 90.9 |
| Mannitol | 24 | 6.3 |
| NaP | 26 | 6.8 |
| MgSO4 | 11 | 2.9 |
| Other | 19 | 4.9 |
SSI, surgical site infection; PEG-ELS, polyethylene glycol-electrolyte lavage solution; NaP, sodium phosphate; MgSO4, magnesium sulfate.
In terms of bowel preparation methods, 56.5% (217/384) Chinese surgeons preferred to choose laxatives alone; laxatives and oral antibiotics was used by 12.0%; laxatives and oral antibiotics combined with enema was used by 20.6%. Enema alone, oral antibiotics alone and other methods were only 7.8%, 0.5% and 2.6%, respectively (Figure 2).
Regarding the choices of agents, Chinese surgeons were more likely to choose PEG-ELS (349/387, 90.9%). Mannitol and NaP were prescribed by 6.3% and 6.8%, respectively. MgSO4 and other agents were only 2.9% and 4.9% (Figure 3).
Analysis of bowel preparation methods
Analysis of bowel preparation methods is summarized in Table 3. The participants’ age and working experience did not show significant differences in the methods of bowel preparation (P value >0.05). In terms of hospitals’ characteristics, there were no significant differences in the use of bowel preparation. Department volume and number of operations also did not significantly affect choosing bowel preparation.
Table 3
| Characteristics | Laxatives | Laxatives and OA | Laxatives, OA and enema | Enema | OA | Other | P value |
|---|---|---|---|---|---|---|---|
| Age | 0.326 | ||||||
| <40 years | 53 | 5 | 17 | 8 | 0 | 1 | |
| 40–50 years | 91 | 25 | 41 | 16 | 1 | 4 | |
| >50 years | 73 | 16 | 21 | 6 | 1 | 5 | |
| Working experience | 0.686 | ||||||
| <10 years | 24 | 1 | 8 | 1 | 0 | 1 | |
| 10–20 years | 59 | 13 | 25 | 10 | 1 | 2 | |
| >20 years | 134 | 32 | 46 | 19 | 1 | 7 | |
| Medical specialty | 0.791 | ||||||
| Gastrointestinal surgery | 57 | 15 | 23 | 12 | 1 | 2 | |
| Anorectal surgery | 77 | 13 | 28 | 6 | 0 | 4 | |
| General surgery | 83 | 18 | 28 | 12 | 1 | 4 | |
| Hospital setting | 0.308 | ||||||
| General | 209 | 44 | 75 | 26 | 2 | 10 | |
| Specialized | 8 | 2 | 4 | 4 | 0 | 0 | |
| Department volume | 0.220 | ||||||
| <50 | 118 | 22 | 43 | 15 | 0 | 4 | |
| 50–100 | 81 | 18 | 27 | 10 | 1 | 5 | |
| 101–150 | 11 | 2 | 4 | 4 | 0 | 1 | |
| >150 | 7 | 4 | 5 | 1 | 1 | 0 | |
| Operations per month | 0.662 | ||||||
| <100 | 142 | 26 | 44 | 17 | 1 | 4 | |
| 100–200 | 60 | 17 | 28 | 11 | 1 | 5 | |
| >200 | 15 | 3 | 7 | 2 | 0 | 1 |
OA, oral antibiotics.
Discussion
Bowel preparation has been controversial for many years, especially in necessity and methods. This survey involved currently the largest number of participants in China. In this study, the characteristics of demographic, hospital and department did not correlate with the methods of bowel preparation. Most of the reasons for bowel preparation were to prevent SSI and avoid anastomotic leakage. Surgeons preferred laxatives when it came to bowel preparation through the data we collected. In terms of laxatives selection, PEG-ELS was mostly used.
Currently, there are only consensuses on bowel preparation before colonoscopy in China, but no guidelines on bowel preparation before colorectal surgery. Chinese surgeons make decisions on management of preoperative bowel preparation for patients based on their own clinical experience or guidelines from other countries. The American Society of Colon and Rectal Surgeons (ASCRS) and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) recommend a combination of MBP and OAP in both colonic and rectal surgery in the 2017 clinical practice guidelines (6). Likewise, the ERAS society guidelines recommended the same methods of bowel preparation (7). However, some countries did not recommend either MBP or MBP + OAP, especially with the spreading of ERAS all over the world. The Canadian guidelines, based on a systematic review of 14 randomized trials and 8 meta-analyses, concluded that it was acceptable to omit MBP as it provided no advantage and might trigger discomfort among patients (8). The British guidelines (updated in 2017 and based on Cochrane reviews) also indicated that stopping or reducing the routine use of MBP in patients undergoing elective colorectal surgery was likely to lead to improved quality of patient care, improved patient experience and productivity savings (9). Furthermore, the French ERAS guidelines did not recommend MBP whether in colonic (strong agreement) or in rectal surgery (weak agreement) (10).
There is a continuous debate on the role of bowel preparation in colorectal surgery. Opponents of MBP argue that it can damage the intestinal mucosal barrier and destroy the endogenous microbial barrier, thereby aggravating the damage of intestinal mucosa directly or indirectly (11). In addition, inadequate MBP caused a significantly higher incidence of peritoneal spillage and subsequent postoperative infectious complications (12). MBP might trigger discomfort among patients, including nausea, vomiting, abdominal pain and abdominal bloating (13).
Regardless of the fact that several studies have shown no benefit from MBP in colorectal surgery, the number of cases they studied was small. The largest and most well-documented study to date came from Kiran’s study (14). They collected NSQIP-targeted colectomy data initiated in 2012 capture information on the use/type of bowel preparation and colorectal-specific complications. Of 8,442 patients, MBP with OAP was independently associated with reduced anastomotic leak [odds ratio (OR) =0.57, 95% confidence interval (CI): 0.35–0.94], SSI (OR =0.40, 95% CI: 0.31–0.53), and postoperative ileus (OR =0.71, 95% CI: 0.56–0.90), compared with MBP alone and no bowel preparation. The findings of this study support the universal adoption of a simple preoperative bowel preparation regimen that combines MBP and oral antibiotics before elective colorectal resection. Bretagnol’s study, the first randomized trial focused on the postoperative results after sphincter-saving rectal resection for cancer, showed that the overall and infectious morbidity rates were significantly higher in no-MBP vs. MBP group, 44% vs. 27%, P=0.018, and 34% vs. 16%, P=0.005, respectively (15).
In our study, most of Chinese surgeons believed that bowel preparation could prevent SSI (81.3%) and avoid anastomotic leakage (74.2%) by reducing the intraluminal bacterial counts and stool burden. In a retrospective study using data from ACS NSQIP Colectomy Targeted database from 2012 to 2015, combined MBP/OAP resulted in significantly lower rates of SSI (OR =0.56, P<0.001) and anastomotic leak (OR =0.53, P<0.001) than no preparation (16). Toh et al. included in their meta-analysis 38 RCTs and 8,458 patients (17). MBP + OAP was better in terms of SSI and wound infection compared to no preparation (OR =0.60, 95% CI: 0.45–0.79 and OR =0.67, 95% CI: 0.48–0.93, respectively), and in terms of SSI and wound infection compared to MBP alone (OR =0.71, 95% CI: 0.57–0.88, and OR =0.62, 95% CI: 0.49–0.85, respectively). Nevertheless, the disruption of the host colonic microbiome and subsequent clostridium difficile infection remains a problem (18). Kim created a propensity-matched analysis of 957 paired cases (n=1,914) and compared patients receiving MBP + OAP with no bowel preparation (19). MBP + OAB group was less likely to develop postoperative clostridium difficile colitis than those who received no bowel preparation (0.5% vs. 1.8%, P=0.01). Bowel preparation should not be omitted considering the benefits above, especially in high-grade colorectal surgery.
Our results showed that Chinese surgeons preferred PEG-ELS as their first laxatives choice (90.9%). PEG-ELS has been widely used for colorectal surgery since 1980. Because of its isotonic and electrolyte-balanced feature, the patient’s electrolytes and internal environment don’t change much, and it has been shown to be highly effective and well tolerated, especially patients with renal insufficiency, congestive heart failure, and advanced liver disease (20). In spite of its safe and adequate cleansing, large volumes of liquid required and unpleasant taste remain troubling patients (21). Modified regimen (a lower-volume PEG formulation, for instance) and improved taste (PEG-ELS-II, for instance) enhance patients’ tolerance with the progress of technology (22,23). Mannitol and NaP are both hyperosmotic cleansing agents which not only can’t be absorbed from the gastrointestinal tract, but also can attract fluid into the bowel. The disadvantage of mannitol is that it can explode by the catabolism of Escherichia coli (24). The preference for NaP has reduced owing to phosphate-induced renal disease (25).
This survey had some limitations. Firstly, the questionnaire did not ask about bowel preparation in colon surgery and rectal surgery separately, which could lead to further findings. In recent years, bowel preparation for colon surgery has often been omitted. This can influence the accuracy of the survey results. Moreover, questionnaire’s options should be more classified in detail. If responders chose ‘other’ on the multiple choice question, they should give a specific description. Finally, there is no discrimination of open or laparoscopic procedures. The questionnaire should include elective and emergency procedures.
Conclusions
In summary, this present survey describes the current attitudes and practice patterns of preoperative bowel preparation among Chinese surgeons. Our study shows that surgeons choose bowel preparation mostly to avoid SSI and anastomotic leakage, and they prefer using laxatives alone. Surgeons do not have clear guidelines that can govern their clinical practice and there are still controversies about bowel preparation. Further study is required to provide strong evidences to inform clinical and policy decisions.
Acknowledgments
We thank the surgeons who kindly participated in the survey.
Funding: This work was supported by the Sanming Project of Medicine in Shenzhen (No. SZSM201911010), the Shenzhen Key Medical Discipline Construction Fund (No. SZXK016), and Guangdong Provincial Key Laboratory of Digestive Cancer Research (No. 2021B1212040006).
Footnote
Reporting Checklist: The authors have completed the SURGE reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-22/rc
Data Sharing Statement: Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-22/dss
Peer Review File: Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-22/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-22/coif). CZ serves as an unpaid Associate Editor-in-Chief of Digestive Medicine Research. All authors report that this work was supported by the Sanming Project of Medicine in Shenzhen (No. SZSM201911010), the Shenzhen Key Medical Discipline Construction Fund (No. SZXK016), and Guangdong Provincial Key Laboratory of Digestive Cancer Research (No. 2021B1212040006). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of The Seventh Affiliated Hospital of Sun Yat-sen University (No. KY-2020-024-01) and individual consent for this cross-sectional survey was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Young H, Knepper B, Moore EE, et al. Surgical site infection after colon surgery: National Healthcare Safety Network risk factors and modeled rates compared with published risk factors and rates. J Am Coll Surg 2012;214:852-9. [Crossref] [PubMed]
- Contant CM, Hop WC, van't Sant HP, et al. Mechanical bowel preparation for elective colorectal surgery: a multicentre randomised trial. Lancet 2007;370:2112-7. [Crossref] [PubMed]
- Güenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2011;CD001544. [Crossref] [PubMed]
- Slim K, Vicaut E, Launay-Savary MV, et al. Updated systematic review and meta-analysis of randomized clinical trials on the role of mechanical bowel preparation before colorectal surgery. Ann Surg 2009;249:203-9. [Crossref] [PubMed]
- Scarborough JE, Mantyh CR, Sun Z, et al. Combined Mechanical and Oral Antibiotic Bowel Preparation Reduces Incisional Surgical Site Infection and Anastomotic Leak Rates After Elective Colorectal Resection: An Analysis of Colectomy-Targeted ACS NSQIP. Ann Surg 2015;262:331-7. [Crossref] [PubMed]
- Migaly J, Bafford AC, Francone TD, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Use of Bowel Preparation in Elective Colon and Rectal Surgery. Dis Colon Rectum 2019;62:3-8. Erratum in: Dis Colon Rectum 2019;62:e436. [Crossref] [PubMed]
- Carmichael JC, Keller DS, Baldini G, et al. Clinical Practice Guidelines for Enhanced Recovery After Colon and Rectal Surgery From the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum 2017;60:761-84. [Crossref] [PubMed]
- Eskicioglu C, Forbes SS, Fenech DS, et al. Preoperative bowel preparation for patients undergoing elective colorectal surgery: a clinical practice guideline endorsed by the Canadian Society of Colon and Rectal Surgeons. Can J Surg 2010;53:385-95. [PubMed]
- 2019 exceptional surveillance of surgical site infections: prevention and treatment (NICE guideline NG125). London: National Institute for Health and Care Excellence (NICE); April 11, 2019.
- Alfonsi P, Slim K, Chauvin M, et al. French guidelines for enhanced recovery after elective colorectal surgery. J Visc Surg 2014;151:65-79. [Crossref] [PubMed]
- Reddy BS, Macfie J, Gatt M, et al. Randomized clinical trial of effect of synbiotics, neomycin and mechanical bowel preparation on intestinal barrier function in patients undergoing colectomy. Br J Surg 2007;94:546-54. [Crossref] [PubMed]
- Mahajna A, Krausz M, Rosin D, et al. Bowel preparation is associated with spillage of bowel contents in colorectal surgery. Dis Colon Rectum 2005;48:1626-31. [Crossref] [PubMed]
- Jung B, Lannerstad O, Påhlman L, et al. Preoperative mechanical preparation of the colon: the patient's experience. BMC Surg 2007;7:5. [Crossref] [PubMed]
- Kiran RP, Murray AC, Chiuzan C, et al. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg 2015;262:416-25; discussion 423-5. [Crossref] [PubMed]
- Bretagnol F, Panis Y, Rullier E, et al. Rectal cancer surgery with or without bowel preparation: The French GRECCAR III multicenter single-blinded randomized trial. Ann Surg 2010;252:863-8. [Crossref] [PubMed]
- Klinger AL, Green H, Monlezun DJ, et al. The Role of Bowel Preparation in Colorectal Surgery: Results of the 2012-2015 ACS-NSQIP Data. Ann Surg 2019;269:671-7. [Crossref] [PubMed]
- Toh JWT, Phan K, Hitos K, et al. Association of Mechanical Bowel Preparation and Oral Antibiotics Before Elective Colorectal Surgery With Surgical Site Infection: A Network Meta-analysis. JAMA Netw Open 2018;1:e183226. [Crossref] [PubMed]
- Krapohl GL, Morris AM, Cai S, et al. Preoperative risk factors for postoperative Clostridium difficile infection in colectomy patients. Am J Surg 2013;205:343-7; discussion 347-8. [Crossref] [PubMed]
- Kim EK, Sheetz KH, Bonn J, et al. A statewide colectomy experience: the role of full bowel preparation in preventing surgical site infection. Ann Surg 2014;259:310-4. [Crossref] [PubMed]
- Seinelä L, Pehkonen E, Laasanen T, et al. Bowel preparation for colonoscopy in very old patients: a randomized prospective trial comparing oral sodium phosphate and polyethylene glycol electrolyte lavage solution. Scand J Gastroenterol 2003;38:216-20. [Crossref] [PubMed]
- Belsey J, Epstein O, Heresbach D. Systematic review: adverse event reports for oral sodium phosphate and polyethylene glycol. Aliment Pharmacol Ther 2009;29:15-28. [Crossref] [PubMed]
- Jansen SV, Goedhard JG, Winkens B, et al. Preparation before colonoscopy: a randomized controlled trial comparing different regimes. Eur J Gastroenterol Hepatol 2011;23:897-902. [Crossref] [PubMed]
- Chen H, Li X, Ge Z. Comparative study on two colonic bowel preparations for patients with chronic constipation. Scand J Gastroenterol 2009;44:375-9. [Crossref] [PubMed]
- Solla JA, Rothenberger DA. Preoperative bowel preparation. A survey of colon and rectal surgeons. Dis Colon Rectum 1990;33:154-9. [Crossref] [PubMed]
- Markowitz GS, Stokes MB, Radhakrishnan J, et al. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol 2005;16:3389-96. [Crossref] [PubMed]
Cite this article as: Yu H, Xu L, Yin S, Hong C, Yang S, Chen J, Li J, Wu W, Zhang C. A Chinese survey of current practice patterns of preoperative bowel preparation in colorectal surgery. Dig Med Res 2022;5:22.