
Diagnosis and management of neonatal gastric perforation: a narrative review
Introduction
Gastric perforation in neonates is an uncommon and often life-threatening clinical entity. It has been estimated to account for approximately 7% of all gastrointestinal perforations (1-3). Siebold first described a case of neonatal gastric perforation in 1825. Herbut reported the first case of a gastric perforation in a newborn with a congenital absence of the musculature within the associated gastric tissue in 1943. However, the first report of an infant successfully treated by surgery for this phenomenon did not occur until 1950 by Leger (4). Linkner and Benson went on to describe a series of 13 newborns with gastric perforation of which 6 survived (4). The literature on this rare entity in the latter half of the twentieth century continued to be dominated by case reports and series with significant speculation on its etiologies in the newborn population. More recent work has been undertaken to understand risk factors and prognostic indicators for clinical outcomes associated with surgical repair of neonatal gastric perforations. Given this disease’s low incidence, the relative significance of these factors is controversial and incompletely understood. In this narrative review, we summarize both the historical context and emerging concepts associated with the possible etiologies, risk factors, clinical presentations, and associated surgical treatment of gastric perforation in neonates. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-105/rc).
Methods
A thorough review of the literature published from 1950 to December, 2021 was completed by independent searches of the publicly available databases the National Institute of Health National Library of Medicine PubMed and MEDLINE for indexed and published articles (Table 1). Editorials and studies written in languages other than English without an accompanying available translation were excluded.
Table 1
| Items | Specification |
|---|---|
| Date of search | December 10, 2021 through December 28, 2021 |
| Databases and other sources searched | National Institute of Health National Library of Medicine PubMed and MEDLINE |
| Search terms used | All combinations of the following terms: “neonatal”, “newborn”, “infant”, “pediatric”, “gastric perforation”, “gastric rupture”, “stomach perforation”, “foregut perforation”, “gastrointestinal perforation” |
| Timeframe | January 1, 1950 to December 31, 2021 |
| Inclusion and exclusion criteria | Inclusion: reports of patients <1 year old with gastric perforation |
| Exclusion: patients 1 year old or older, studies written in languages other than English without an accompanying translation, reports of any perforation not involving a gastric perforation | |
| Selection process | Both authors |
Etiology
The etiology of gastric perforations in newborns remains to be fully elucidated. Iatrogenic causes such as from forceful naso- or orogastric tube insertion have been documented in several cases (5-8). Historically, many authors proposed that a congenital defect in the musculature of the gastric wall may explain the occurrence of these ruptures (9-13). Specifically, histopathology of gastric tissue has been reported to show thin or even complete absence of longitudinal muscular fibers in areas of perforation (9-11,13). More recent pathological work has implicated other histological changes such as a decrease in the number of interstitial cells of Cajal in areas of perforated stomach musculature (14). These explanations fail to serve as a fully unifying explanation of this phenomenon as many reports demonstrate musculature present in ruptured segments (15,16). Furthermore, experimental studies have been undertaken providing evidence against the concept of a congenital basis for gastric perforation. In 1965, Shaw et al. (17) tested gastric distensibility in a canine model by ligating the esophagus and duodenum and insufflating the stomach until gastric rupture occurred. All perforations were located on the greater curvature, and subsequent pathology in all cases demonstrated a lack of musculature in the area of rupture. From these findings, the authors concluded that an absence of muscle fibers may be a nonspecific result of perforation itself as opposed to its pathological etiology (17). Other preclinical models in rodent newborns utilizing air insufflation along with intragastric barium suggest these factors may create a “fluid-air trap” resulting in a physiologic gastric outlet obstruction as well as preventing reflux of air in to the esophagus during feeds that can result in perforation (18). The authors hypothesized that this mechanism may be able to occur in normal infants who can accumulate enough air in the stomach during feeds to cause gastric rupture (18). Similarly, others have hypothesized that associated gastrointestinal anomalies such as tracheoesophageal fistula, duodenal web, duodenal atresia, congenital diaphragmatic hernia, hiatal hernia, or malrotation can lead to increased intragastric pressure and resultant perforation (16,19-23).
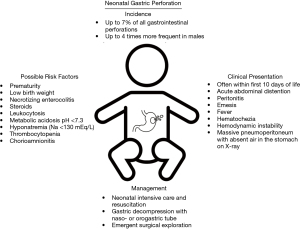
Another theory for neonatal gastric perforation is local hypoxia and ischemic insult to the gastric tissue. Touloukian et al. (24) in 1972 utilizing an experimental model of neonatal piglets demonstrated that a significant reduction in mucosal blood flow to the stomach, distal ileum and colon can occur during asphyxia with resultant areas of necrosis. Touloukian went on to further investigate a series of six infants with gastric perforation treated within his own institution as well as 87 previous cases from the literature and determined that 69% of cases had perinatal complications resulting in asphyxia (25). This asphyxia and local tissue hypoxia when combined with other contributing factors such as hypotension, sepsis, high gastric acidity or trauma can be a significant contributing factor to gastric ischemia and perforation (15,16,25-31). Some have even argued that the degree of hypoxia and these contributing factors can determine the severity of gastric necrosis and perforation encountered intraoperatively (32). Despite this evidence, the exact etiology of neonatal gastric perforation remains to be elucidated but is likely a combination of many factors worsened by increased intragastric pressure and hypotensive or hypoxic states (Figure 1).
Risk factors
Given these possible explanations for the underlying causes of neonatal gastric perforation, recent work has shifted to focus on associated risk factors for its development. Two of the most well recognized risk factors uncovered among infants with this disease are prematurity and low birth weight. Many reports of premature infants have been documented in the literature presenting with gastric perforation with this population accounting for the majority of cases compared to gestational age infants in some series (23,29,33-36). Furthermore, it has been suggested that gastric perforations in premature infants may be associated with worse clinical outcomes and higher mortality compared to those born at full term (23,29,35,36). Similar to prematurity, low birth weight has been shown in many studies to be related to a propensity for developing gastric perforation especially among low and extremely low birth weight infants (21,28,34,35,37). While the relationship between prematurity, birth weight and the development of necrotizing enterocolitis has been often described, the association of necrotizing enterocolitis and risk of gastric perforation in the neonatal period has also been demonstrated in some series (23,38,39). In these cases, mortality is higher among cases of gastric perforation associated with necrotizing enterocolitis (38,39). Among full term infants, steroid therapy has been described in one report as a risk factor for gastric rupture but has not been widely cited as a factor in other studies (21).
Similar to previous explanations demonstrating aerophagia and increased gastric distention worsening the risk of perforation (17), the need for mechanical ventilation and specifically nasal ventilation in one study has been suspected to be a risk factor for this phenomenon (15,16). Some studies have also demonstrated an unequal sex distribution of this disease occurring as high as four times more frequently in males compared to female infants (28,40). Other unfavorable prognostic clinical factors found to be associated with gastric perforation are leukocytosis, metabolic acidosis (pH <7.3), hyponatremia (serum sodium <130 mEq/L), thrombocytopenia, hypotension, and chorioamnionitis (15,19,21,28,33-36,41). Taken together, the presence of these factors, especially prematurity and low birth weight, should raise suspicion for the development of gastric perforation in the neonatal period and escalate the degree of clinical monitoring in these infants (Figure 1).
Clinical presentation
The onset of gastric perforation often occurs early in the neonatal period even as early as within hours after birth (7). The majority of reports demonstrate symptom onset within the first ten days of life (32-34,42); however, perforation can occur outside of this time frame (32). One of the most common and consistent clinical findings of neonatal gastric perforation is massive acute onset abdominal distention as high as 87% in some series (7,15,19,27). Emesis has also been noted to occur in as many as 40% of patients presenting with gastric perforation (15). Peritonitis is often present on exam, and other clinical signs may include lethargy, hemodynamic instability, hematochezia, hematemesis, and fever (4,8,15,16,19,23,29,34,41,43,44). Radiographic findings on upright abdominal X-ray often demonstrate massive pneumoperitoneum with the absence of gas in the stomach referred to as a “football” or “saddle bag” sign (7,8,19,39,42). Radiographic pneumoperitoneum has been documented in as many as 86% of patients in some reports (15). Portal venous gas may or may not be present as well on abdominal radiographs (38). Laboratory values may demonstrate leukocytosis, metabolic acidosis, hyponatremia, lactatemia, thrombocytopenia, hypotension, and elevated inflammatory markers such as C reactive protein (15,19,21,28,33-36). Once a diagnosis of gastric perforation is made, expeditious resuscitation efforts and surgical consultation should be performed (Figure 1).
Management and outcomes
Once gastric perforation has been diagnosed, surgical exploration is mandated. Preoperatively, fluid resuscitation and hemodynamic monitoring and support should be initiated if not already underway. In select cases, preoperative peritoneocentesis has been utilized as both a diagnostic and therapeutic tool for decompression of the abdomen particularly in patients with significant respiratory distress and hemodynamic compromise from massive pneumoperitoneum (2,45,46). Gastric decompression with placement of a naso- or orogastric tube should be undertaken to improve intramural blood flow by decreasing distensibility of the stomach (23).
The most common surgical approach includes laparotomy with careful inspection of the entire stomach. Although other locations such as the fundus and lesser curvature can be involved, the majority of cases report the perforation site most commonly on the greater curvature of the stomach accounting for as many as 85–95% of perforations in some series (17,28,39,40,47,48). Perforation size may often be between 1 to 5 cm and linear or circular appearing (28,40,45,47). Perforations resulting from iatrogenic trauma such as nasogastric tube insertion have been described as having a “punched out” appearance (2). If no significant concomitant ischemic or necrotic tissue is encountered, debridement and primary repair has been successfully completed in many cases (15,16). Rarely, cases have been described in which such a large degree of necrotic gastric tissue was encountered that required partial or total gastrectomy (41,49-53). Severe cases requiring total gastrectomy have also described utilizing Hunt-Lawrence pouches as an option for immediate or delayed gastric reconstruction (51-53). Some surgeons routinely perform gastrostomy tube at the time of perforation in anticipation of postoperative nutritional support; however, the effect of routine gastrostomy tube placement on postoperative outcomes is incompletely characterized (40). Laparoscopic repair of single gastric perforations has been documented successfully in two cases, which may hold the advantages of minimally invasive surgery including reduced postoperative pain, analgesic requirement, and decreased length of stay in appropriately selected patients (54).
Traditionally, neonatal gastric perforation has been associated with substantial mortality rates as high as 62–70% and higher (16,28). However, recent work stratifying reports of this phenomenon by decade has shown an interval decrease from nearly 100% case fatality in 1980 to 17% by 2016 (15). Recent advancements in both operative and neonatal postoperative intensive care have been cited as reasons for improvement in the clinical outcomes of these patients (15,19,39). Enhancements in diagnostic capabilities in the past few decades have allowed for earlier diagnosis and improved resuscitation preoperatively, which can result in better outcomes (16). Despite these advancements in the treatment of and mortality associated with gastric perforation, it continues to persist as a life-threatening entity and should be treated as a surgical emergency in the neonatal population.
Conclusions
Overall, gastric perforation is an uncommon yet life-threatening clinical entity in neonatal patients. Although significant advancements in its diagnosis and treatment have been achieved in the past few decades, its underlying pathogenesis remains to be fully characterized. For nontraumatic cases, previous work has attempted to describe a mechanistic basis for these perforations such as from a congenital agenesis in the stomach musculature or hypoxic-ischemic insult to the gastric tissue. However, no singular, unifying theory has emerged. While studies have been undertaken to understand prognostic risk factors associated with neonatal gastric perforation such as low birth weight and prematurity, future work should be undertaken to delineate prognostic factors or clinical scoring systems to direct early monitoring and intensive care to at-risk infants. Despite significant improvements in neonatal resuscitation and surgical therapy in the last few decades, gastric perforation is still associated with substantial morbidity and mortality in newborns and should be treated as an emergency once diagnosed prompting urgent pediatric surgical consultation. Areas of further study regarding its management should include comparison of procedural techniques to optimize postoperative outcomes in these patients including when peritoneocentesis should be performed and whether gastrostomy tubes should be placed routinely at the time of surgery. Although significant strides in diagnosis and treatment continue to be made, much remains to be studied in the future to improve outcomes for gastric perforation in this vulnerable patient population.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-105/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-105/coif). EAP serves as an unpaid editorial board member of Digestive Medicine Research form March 2021 to February 2023. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Attridge JT, Clark R, Walker MW, et al. New insights into spontaneous intestinal perforation using a national data set: (2) two populations of patients with perforations. J Perinatol 2006;26:185-8. [Crossref] [PubMed]
- Jawad AJ, Al-Rabie A, Hadi A, et al. Spontaneous neonatal gastric perforation. Pediatr Surg Int 2002;18:396-9. [Crossref] [PubMed]
- Aydin M, Zenciroğlu A, Hakan N, et al. Gastric perforation in an extremely low birth weight infant recovered with percutaneous peritoneal drainage. Turk J Pediatr 2011;53:467-70. [PubMed]
- Linkner LM, Benson CD. Spontaneous perforation of the stomach in the newborn; analysis of thirteen cases. Ann Surg 1959;149:525-33. [Crossref] [PubMed]
- Ebenezer K, Bose A, Carl S. Neonatal gastric perforation following inadvertent connection of oxygen to the nasogastric feeding tube. Arch Dis Child Fetal Neonatal Ed 2007;92:F407. [Crossref] [PubMed]
- Sinnathamby A, Low JM, Dale Lincoln Ser Keng L, et al. Watch your numbers! Avoiding gastric perforation from feeding tubes in neonates. Pediatr Neonatol 2021;62:681-2. [Crossref] [PubMed]
- Sadat AS, Thotan SP, Prabhu SP, et al. Large Sealed Neonatal Gastric Perforation: A Case Report. Clin Pediatr (Phila) 2019;58:1321-3. [Crossref] [PubMed]
- Saraç M, Bakal Ü, Aydın M, et al. Neonatal Gastrointestinal Perforations: the 10-Year Experience of a Reference Hospital. Indian J Surg 2017;79:431-6. [Crossref] [PubMed]
- Braunstein H. Congenital defect of the gastric musculature with spontaneous perforation: Report of five cases. J Pediatr 1954;44:55-63. [Crossref] [PubMed]
- MacGillivray PC, Stewart AM, MacFarlane A. Rupture of the stomach in the newborn due to congenital defects in the gastric musculature. Arch Dis Child 1956;31:56-8. [Crossref] [PubMed]
- Meyer JL 2nd. Congenital defect in the musculature of the stomach resulting in spontaneous gastric perforation in the neonatal period; a report of two cases. J Pediatr 1957;51:416-21. [Crossref] [PubMed]
- Inouye WY, Evans G. Neonatal gastric perforation. A report of six cases and a review of 143 cases. Arch Surg 1964;88:471-85. [Crossref] [PubMed]
- Amadeo JH, Ashmore HW, Aponte GE. Neonatal gastric perforation caused by congenital defects of the gastric musculature. Surgery 1960;47:1010-7. [PubMed]
- Jactel SN, Abramowsky CR, Schniederjan M, et al. Noniatrogenic neonatal gastric perforation: the role of interstitial cells of Cajal. Fetal Pediatr Pathol 2013;32:422-8. [Crossref] [PubMed]
- Yang T, Huang Y, Li J, et al. Neonatal Gastric Perforation: Case Series and Literature Review. World J Surg 2018;42:2668-73. [Crossref] [PubMed]
- Leone RJ Jr, Krasna IH. 'Spontaneous' neonatal gastric perforation: is it really spontaneous? J Pediatr Surg 2000;35:1066-9. [Crossref] [PubMed]
- Shaw A, Blanc WA, Santulli TV, et al. Spontaneous Rupture of the Stomach in the Newborn: A Clinical and Experimental Study. Surgery 1965;58:561-71. [PubMed]
- James AE Jr, Heller RM Jr, White JJ, et al. Spontaneous rupture of the stomach in the newborn: clinical and experimental evaluation. Pediatr Res 1976;10:79-82. [Crossref] [PubMed]
- Yang CY, Lien R, Fu RH, et al. Prognostic factors and concomitant anomalies in neonatal gastric perforation. J Pediatr Surg 2015;50:1278-82. [Crossref] [PubMed]
- Terui K, Iwai J, Yamada S, et al. Etiology of neonatal gastric perforation: a review of 20 years' experience. Pediatr Surg Int 2012;28:9-14. [Crossref] [PubMed]
- Duran R, Inan M, Vatansever U, et al. Etiology of neonatal gastric perforations: review of 10 years' experience. Pediatr Int 2007;49:626-30. [Crossref] [PubMed]
- Akcora B, Eris O. A newborn with duodenal atresia and a gastric perforation. Afr J Paediatr Surg 2010;7:33-5. [Crossref] [PubMed]
- Kuremu RT, Hadley GP, Wiersma R. Neonatal gastric perforation. East Afr Med J 2004;81:56-8. [Crossref] [PubMed]
- Touloukian RJ, Posch JN, Spencer R. The pathogenesis of ischemic gastroenterocolitis of the neonate: selective gut mucosal ischemia in asphyxiated neonatal piglets. J Pediatr Surg 1972;7:194-205. [Crossref] [PubMed]
- Touloukian RJ. Gastric ischemia: the primary factor in neonatal perforation. Clin Pediatr (Phila) 1973;12:219-25. [Crossref] [PubMed]
- Chen TY, Liu HK, Yang MC, et al. Neonatal gastric perforation: a report of two cases and a systematic review. Medicine (Baltimore) 2018;97:e0369. [Crossref] [PubMed]
- Iacusso C, Boscarelli A, Fusaro F, et al. Pathogenetic and Prognostic Factors for Neonatal Gastric Perforation: Personal Experience and Systematic Review of the Literature. Front Pediatr 2018;6:61. [Crossref] [PubMed]
- Babayigit A, Ozaydın S, Cetinkaya M, et al. Neonatal gastric perforations in very low birth weight infants: a single center experience and review of the literature. Pediatr Surg Int 2018;34:79-84. [Crossref] [PubMed]
- Lin CM, Lee HC, Kao HA, et al. Neonatal gastric perforation: report of 15 cases and review of the literature. Pediatr Neonatol 2008;49:65-70. [Crossref] [PubMed]
- McAleese JJ, Sieber WK. The surgical problem presented by peptic ulcer of the stomach and duodenum in infancy and childhood. Ann Surg 1953;137:334-41. [Crossref] [PubMed]
- Beattie JW, Bohan KE. Perforation of gastric ulcer in premature newborn with operation and survival. Am Surg 1952;18:1146-9. [PubMed]
- Garge SS, Paliwal G. Neonatal Gastric Perforation: Our Experience and Important Preoperative and Intraoperative Caveats to Prognosticate and Improve Survival. J Indian Assoc Pediatr Surg 2020;25:201-5. [Crossref] [PubMed]
- Abdelgawad AEM, Darwish AA, Hughes E, et al. Spontaneous Gastric Perforation in Neonates: A Tertiary Pediatric Surgical Center Experience. J Neonatal Surg 2019;8:20. [Crossref]
- Bruce J, Bianchi A, Doig CM, et al. Gastric perforation in the neonate. Pediatr Surg Int 1993;8:17-9. [Crossref]
- Huang Y, Lu Q, Peng N, et al. Risk Factors for Mortality in Neonatal Gastric Perforation: A Retrospective Cohort Study. Front Pediatr 2021;9:652139. [Crossref] [PubMed]
- Byun J, Kim HY, Noh SY, et al. Neonatal gastric perforation: A single center experience. World J Gastrointest Surg 2014;6:151-5. [Crossref] [PubMed]
- Sato M, Hamada Y, Kohno M, et al. Neonatal gastrointestinal perforation in Japan: a nationwide survey. Pediatr Surg Int 2017;33:33-41. [Crossref] [PubMed]
- Sakellaris G, Partalis N, Dede O, et al. Gastrointestinal perforations in neonatal period: experience over 10 years. Pediatr Emerg Care 2012;28:886-8. [Crossref] [PubMed]
- Hashim I, Talat N, Iqbal A, et al. Spontaneous gastric perforation: is it really common? Ann Pediatr Surg 2021;17:13. [Crossref]
- Rosser SB, Clark CH, Elechi EN. Spontaneous neonatal gastric perforation. J Pediatr Surg 1982;17:390-4. [Crossref] [PubMed]
- Sakaria RP, Zaveri PG. Neonatal Gastric Perforation: 14-Year Experience from a Tertiary Neonatal Intensive Care Unit. Am J Perinatol 2021; Epub ahead of print. [Crossref] [PubMed]
- Bayatpour M, Bernard L, McCune F, et al. Spontaneous gastric rupture in the newborn. Am J Surg 1979;137:267-9. [Crossref] [PubMed]
- Milassin TG, Ng ZQ, Gera P. Haematemesis as a sign of silent neonatal gastric perforation. J Paediatr Child Health 2020;56:1830-2. [Crossref] [PubMed]
- Shashikumar VL, Bassuk A, Pilling GP IV, et al. Spontaneous gastric rupture in the newborn: a clinical review of nineteen cases. Ann Surg 1975;182:22-5. [Crossref] [PubMed]
- Parrish RA, Sherman RT, Wilson H. Spontaneous Rupture of the Gastro-enteric Tract in the Newborn A Report of 13 Cases and Description of a Characteristic X-ray Finding. Ann Surg 1964;159:244-51. [Crossref] [PubMed]
- Nagaraj HS, Sandhu AS, Cook LN, et al. Gastrointestinal perforation following indomethacin therapy in very low birth weight infants. J Pediatr Surg 1981;16:1003-7. [Crossref] [PubMed]
- Houck WS Jr, Griffin JA 3rd. Spontaneous linear tears of the stomach in the newborn infant. Ann Surg 1981;193:763-8. [Crossref] [PubMed]
- Wang K, Cai S, He L, et al. Pediatric gastric perforation beyond neonatal period: 8-year experience with 20 patients. Pediatr Neonatol 2019;60:634-40. [Crossref] [PubMed]
- Quak SH, Joseph VT, Wong HB. Neonatal total gastrectomy. Clin Pediatr (Phila) 1984;23:507-8. [Crossref] [PubMed]
- Marrie V, Moosa A, Cywes S. Gastric perforation in the newborn. S Afr Med J 1978;54:202-4. [PubMed]
- Theodorou CM, Chen P, Vanover MA, et al. Total gastrectomy with delayed Hunt-Lawrence pouch reconstruction for neonatal gastric perforation presenting with hematemesis. J Pediatr Surg Case Rep 2020;63:101686. [Crossref] [PubMed]
- Durham MM, Ricketts RR. Neonatal gastric perforation and necrosis with Hunt-Lawrence pouch reconstruction. J Pediatr Surg 1999;34:649-51. [Crossref] [PubMed]
- Govind SK, Livingston M, Flageole H, et al. Gastric necrosis in a term infant treated with near-total gastrectomy and delayed reconstruction with a Hunt-Lawrence pouch. BMJ Case Rep 2019;12:231869. [Crossref] [PubMed]
- Glüer S, Schmidt AI, Jesch NK, et al. Laparoscopic repair of neonatal gastric perforation. J Pediatr Surg 2006;41:e57-8. [Crossref] [PubMed]
Cite this article as: Huerta CT, Perez EA. Diagnosis and management of neonatal gastric perforation: a narrative review. Dig Med Res 2022;5:27.


