
A painful pandemic: review of the opioid crisis and major surgery
Introduction
Alongside hypnosis and muscle relaxation, analgesia makes up the triad of a ‘balanced’ anaesthetic. Opioids have long been a cornerstone of providing effective analgesia in the perioperative period. Over the last ten years rates of acute opioid prescriptions have risen dramatically, despite an ever-increasing body of evidence relating to major side effects, addiction, and increased morbidity and mortality (1). In the United Kingdom (UK), there has been a four-fold increase in opioid prescriptions over the past decade, with codeine-containing medications being the most commonly prescribed. Alarmingly, this trend in prescriptions is mirrored by an upward spiral of opioid-related dependence and associated fatalities (2,3).
The scale of this healthcare crisis is well documented and whilst the United States of America (USA) may be visible as the epicentre, it is clear the effects of opioid over-prescription and the unintended diversion of medication are further reaching. In 2017, 110,000 people died globally secondary to opioid-use disorders, a 77% increase compared to the previous decade (4). The following year, in the USA alone, 68,500 people died from opioid overdoses and an estimated 2 million people had a diagnosis of an opioid use disorder (5,6).
As surgical patients are nearly four times more likely to receive a prescription containing opioids than non-surgical patients, it is not surprising that the perioperative period has been demonstrated as an important source of new persistent opioid use (1,5). Addressing this issue further is challenging as there is no universally agreed definition of persistent postoperative opioid use (PPOU). Indeed, Jivraj et al. (7) identified 29 different definitions existing across 39 different studies. However, all of these relate PPOU to ongoing opioid consumption beyond the point at which postoperative pain would normally have been expected to have resolved. This time frame varies dramatically between studies, from 90 days to 1 year and therefore, as expected, the suggested prevalence also varies. Higgins et al. (8) undertook a systematic review and meta-analysis to estimate the incidence of opioid dependency following surgery in patients who had previously been opioid-naive. The pooled incidence was 4.7% with confidence intervals of 2.1–10.4%, but the researchers admitted significant heterogeneity between the 12 studies identified for analysis. Other review articles suggested similar rates of chronic opioid use following surgery (3–10%) (1,9-12).
Unfortunately, the coronavirus-19 (COVID-19) pandemic has likely served to exacerbate chronic opioid usage, in the wake of mass surgical cancellations ranging from elective pain-relieving procedures such as joint replacements through to urgent major oncological surgery. It is becoming increasingly clear that ‘the opioid epidemic’ might be more aptly renamed ‘the opioid pandemic’. The anaesthetic and surgical teams have a major role to play in appropriately limiting opioid use in the perioperative period, thus hopefully reducing the healthcare and financial burden associated with subsequent long-term opioid consumption.
This review summarises some of the recent research into the opioid crisis and PPOU. It discusses the influence of patient traits and prescriber habits and the drive towards opioid-sparing surgery, including options for multimodal analgesia and enhanced recovery after surgery (ERAS) programs.
History of the opioid crisis
Providing optimal analgesia during the perioperative period underpins the safe and effective management of anaesthesia and surgery. However, efforts to improve patient satisfaction surrounding pain management in the recent past have since been shown to be a major contributor to over-prescription of opioid medications, unwittingly fuelling the fire that has become the opioid crisis.
During the mid-1990s in the USA, in efforts to improve the inadequacies in pain management, the American Pain Society advised that pain should be assessed with the same consideration as other key vital signs and that severe pain should be seen as a “red flag” needing urgent management. A few short years later, the concept of pain as the “fifth vital sign” was born, with a greater emphasis on education surrounding pain assessment, mandated documented pain assessment scores and early aggressive treatment (1,2).
The use of subjective scores relating to patients’ experiences of pain and its management by practitioners, increasingly became part of quality control data used to measure healthcare standards. Critically, satisfaction surveys utilised by the Centres for Medicare and Medicaid Services helped to calculate the financial reimbursement of US healthcare providers; the survey worded the question “How often did the hospital or provider do everything in their power to control your pain?”. Suggestions have been made that this question and its influence over healthcare provider income, had the inadvertent consequence of encouraging opioid over-prescription (2).
Prescription of opioids in this period went relatively unchecked, in part due to a lack of awareness and misinformation about the addictive nature of opioids and thus the perceived low risk of abuse (5). Inconsistent insurance cover for non-opioid and more holistic approaches to pain management has been an additional factor in accelerating the prevalence of opioid prescriptions (5). However, arguably the biggest influence was the aggressive advertising techniques seen by pharmaceutical companies aimed at both patients and practitioners (5). A damning article, Marks 2020 (13) accuses several opioid companies of engaging in corporate strategies to increase prescription of opioids to large patient groups (both cancer and non-cancer) as well as to downplay concerns over opioid addiction (13). These strategies, similar to those employed by tobacco companies, have been born from public relations and management consultancy and now appear embedded at every level of the system from government to society, having had influence over medical practice, medical research and public health policy (13).
Although it was not until more recently that the dangers of an unmoderated approach to opioid prescription came into focus (5), it could be dangerous to think that opioid manufactures no longer have such an influential stake in the game. One such example of this was revealed in 2019 when the World Health Organisation (WHO) discontinued two of its guidelines: “Ensuring Balance in National Policies on Controlled Substances” and “WHO Guidelines on the Pharmacological Treatment of Persisting Pain in Children with Medical Illnesses”. This occurred following an investigation prompted by allegations that these guidelines were tainted by the influence of opioid companies (14). While the WHO argued that these guidelines needed updating, it acknowledged that removing them would resolve any conflicts of interest from the experts who wrote them (14). The extent of the influence of opioid manufactures on the opioid crisis is beyond the scope of this article but cannot, in our opinion, be ignored.
Changing the ethos of pain
Traditional approaches to pain assessment include unidimensional, numerical, self-reported scores including linear scales of ‘0–10’ or ‘mild to severe’. Given the lack of evidence of improved outcomes following implementation of these numerical pain scores, along with increased rates of opioid over-sedation, The Joint Commissions in the US has now stressed the importance of assessing pain in the context of its effect on patient function and ability of patients to work towards their treatment goals (2).
With this more dynamic approach in mind, The American Pain Society now recommends the use of novel scoring systems such as the Functional Activity Score (FAS). The aim is to provide optimal instead of complete analgesia and to assess adequacy of pain control to enable the patient to undertake appropriate activities during the postoperative period (1,2). For example, following major abdominal surgery, a patient should be expected to deep breathe, cough and move in bed in early recovery, but not mobilise until day one or two postoperatively. This approach has partly underpinned new enhanced recovery protocols, attempting to limit the use of opioid medications to facilitate achievement of predefined rehabilitation goals.
Clear communication, preparation and education around perioperative pain is vital to managing patient expectations. Soffin et al. (5) and Koepke et al. (1) recommend educating patients and their families in the preoperative setting, to clarify the pain they may expect to experience, management techniques, the dangers of opioid dependence and addiction, and the safe disposal and storage of medication. Preoperative anaesthetic clinic visits provide an optimal opportunity to discuss multimodal and regional anaesthetic techniques that form part of the patient’s individual pain plan. The key to success may involve temporising patient expectations of a pain-free postoperative recovery. Educating patients in this manner has been associated with improved clinical outcomes and may reduce opioid demand in the perioperative period. A new approach to empowering patients to take more responsibility for reducing their own risk of PPOU was utilised by the Toronto General Hospital; their transitional pain service included a signed opioid contract in the post-discharge pain management plan (1,5).
Patient risk factors associated with PPOU
Early identification of patients at risk of PPOU is a key strategy in reducing its ongoing prevalence. A review article investigating associated patient characteristics reported a higher risk of PPOU in those with perioperative benzodiazepine and selective serotonin reuptake inhibitor (SSRI) use, diabetes and lower socioeconomic backgrounds (1). Other articles have highlighted depression, anxiety, substance abuse and smoking as key risk factors (3,15). Age and gender may also be risk factors, though there seems little agreement between studies of how these are specifically associated with increased risk (1,15,16).
Despite minimal consensus over the varying patient risk factors and a paucity of information regarding the relative importance of these, one of the most significant predictors of PPOU is chronic preoperative opioid use (9,15). Studies found the incidence of PPOU across a range of different surgical procedures in this patient group was between two- and ten-fold greater, compared with opioid-naïve patients (3).
Critically, it has also been suggested that chronic preoperative opioid use is associated with poorer surgical outcomes and worse morbidity and mortality (5,15). In a large cohort study, 8.8% of elective patients used opioids prior to surgical intervention (16). This group had longer inpatient stays and were more likely to require readmission (16). Gerrish et al. (9) also report an association between preadmission opioid use and greater postoperative pain, longer recovery time, poorer postoperative function and increased prevalence of complications.
Unfortunately, the overall benefit of risk factor modification remains unclear (9). However, identification of patients with chronic preoperative opioid use presents an opportunity to instigate preoperative opioid weaning, which could be associated with improved surgical outcomes. Interestingly, in patients suffering from depression, improving compliance with antidepressants has been associated with reduced preoperative opioid use. This could be in part due to a reduction in the emotional stress associated with the pain response (5). The introduction of ERAS pathways provides an ideal opportunity to create a patient-centred analgesic plan and target identified modifiable risk factors.
Understanding the type of prescriptions that risk dependence
There is a well-documented correlation between duration, dose, and number of repeat prescriptions of postsurgical opioids and the risk of PPOU, along with other forms of opioid misuse. A retrospective analysis of opioid-naive, cancer-free patients from a large US insurance database found that the overall incidence of long-term use (>1 year) following the first opioid prescription was 6.0% (17). However, the risk dramatically increased with the duration of the initial prescription. For those who received a prescription of 8 days or more, the incidence of long-term use more than doubled to 13.5%, and doubled again to 29.9% in prescriptions dispensing greater than a 30-day supply (17).
This same study by Shah et al. (17), as well as a retrospective cohort study conducted by Brat et al. (11), further found that a greater total number of repeat prescriptions dispensed, increased the risk of PPOU. In fact, Brat et al. (11), reported that it was this, and not the duration of prescriptions, that was the best predictor for opioid misuse. In the study’s large cohort, 56% filled a prescription for opioids in the postoperative period. Despite the overall incidence of misuse being low at 0.6%, the rate of misuse more than doubled among patients who repeated their initial prescription. After adjustment for covariates, the rate of misuse increased by 44% for every repeat prescription refilled thereafter.
As expected, the total dose of opioid is related to long-term use. Shah et al. (17), reported that total doses of greater than 700 morphine milligram equivalents (MME) were associated with higher occurrence of long-term opioid use. Brat et al. (11) concurred with this suggestion, but identified a weaker association with total dose than with the duration of opioid prescriptions. Importantly, the combination of a long duration of use and higher dose of opioid prescriptions had a dramatic effect, with the rate of misuse sharply increasing for those receiving higher-dose opioids for longer than 9 weeks (11).
As such, it is reasonable to expect that restriction in duration of postoperative opioid prescriptions, during admission and after discharge, would improve long-term opioid usage (10). This suggestion has been echoed by many authors. Shah et al. (17) specifically recommends that when initiating opioids, prescriptions should be for less than 7 days, and ideally less than 3 days. In addition, opioid-naïve patients should be advised to abstain from repeating or refilling prescriptions following hospital discharge (10).
Overprescription of postoperative opioids is apparent in multiple surgical specialties. Several articles have reported that a significant proportion (42–80%) of prescribed opioids are not utilised and patients often require fewer doses than is anticipated (1,5,11). Concerningly, when surveyed, few patients reported receiving advice on how to safely store or dispose of these medications and over 70% admitted their prescription opioids were not kept securely (1,5). A by-product of opioid over-prescription is diversion of these addictive medications, whereby opioids find their way into non-prescription markets (18). Even more worryingly, a survey found that 75% of heroin users began their addiction by taking prescribed opioid medication that was often intended for someone else (19). Lipari and Hughes (20) [2017] reported that approximately 50% of those who had misused prescription drugs in the preceding year had obtained them, free of charge, from a friend or relative. Eliminating or even reducing over-prescription of opioids should, in theory, also reduce diversion.
The evident need for safe and secure prescribing and dispensing of opioid medication has led to investment and research into innovative technological solutions. One such product, Pilleve, is currently undergoing clinical trials aimed at regulating the timed delivery of controlled medications in a tamper-proof container. This is digitally linked to patient pain scores, reported side effects and a doctor-patient portal which can be closely monitored. This could assist in identifying opioid misuse at an earlier stage (21).
Improving clinician prescriber habits
Considering the significant public attention the opioid epidemic has received in recent years, and the central role healthcare and allied healthcare professionals play in a patient’s access to opioid medication in the perioperative period, healthcare staff receive little education on the topic (5). Poor opioid stewardship results in opioid dependence, misuse and diversion following discharge (10).
Modifying prescriber habits through clinician education, as well as through the implementation of national and local guidelines, may improve safety with regards to opioid prescriptions; Figure 1 outlines suggestions for healthcare workers to reduce PPOU in surgical patients. To date, several institutions have made efforts to achieve this on local or regional scales, some with promising results. For instance, in the US, the state of Florida instituted a series of major policy changes that were designed to reduce the inappropriate supply of prescription opioids. After these policies were implemented, prescriptions were curtailed, and the rate of death from prescription-opioid overdose declined 27% between 2010 and 2012 (19). In another example, the Michigan Opioid Prescribing Engagement Network (Michigan OPEN), a collaboration of hospitals, doctors and insurers, established surgery-specific guidelines for postoperative opioid prescribing for common procedures following a state-wide study involving nearly 12,000 patients across 43 hospitals (22). Seven months after these were published, they identified close to a 30% reduction in the number of tablets prescribed, with no reported decrease in patient satisfaction with pain management (22).
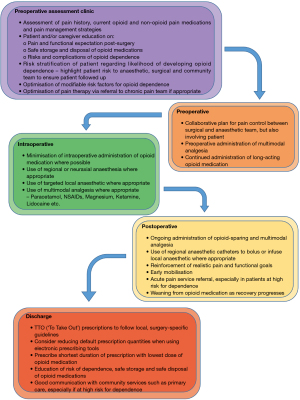
A promising strategy to implement real change may be through ERAS programs. These have been implemented across a variety of surgical specialties in recent years with the aim of attenuating the surgical stress response and standardising perioperative care to improve patient outcomes and recovery (9). As a multidisciplinary, patient-centred pathway, they provide the framework for ensuring surgery-specific guidelines are followed with regards to inpatient and outpatient opioid prescription. In fact, they often instigate protocols which promote opioid-free and multimodal analgesia, with an emphasis on non-steroidal medications, neuroleptic modulators, N-methyl-D-aspartate (NMDA) receptor antagonists, and regional or neuraxial anaesthesia (22,23).
However, the motive for decreasing perioperative opioids seemingly has less to do with dependence and more to do with supporting early mobilisation and function, thus promoting an expedited discharge. As such, while their impact on reducing inpatient opioid use may be profound, this often does not apply to discharge and outpatient prescriptions. For example, a study of patients undergoing colorectal surgery on the ERAS pathway at an academic tertiary hospital, demonstrated a reduction in inpatient opioid use and shorter length of stays, but a statistically significant increase in opioid prescribing at and within the first 30 days post-discharge (9). An alternative study by Brandal et al. (23) found that implementation of an ERAS intervention did not significantly lower the rate of opioid prescription at discharge. It found that for patients with a low discharge pain score, no preoperative opioid use and low morphine consumption prior to discharge, the rate of opioid prescription at discharge was high at 72% (23). In order to reduce PPOU there must be a clear focus on reducing opioid prescriptions on and after discharge. It is plausible that this could happen with changes to the ERAS framework.
The adaptation of electronic prescribing in hospitals may be another effective way of altering prescriber habits to reduce opioid over-prescription at discharge. Research published in 2018 led by Yale University, assessed if reducing the prescription dose and duration defaults for postoperative opioids in electronic medical record (EMR) systems, led to improved opioid prescribing practices. Fascinatingly, their study demonstrated a greater than 15% decrease in opioid prescriptions across the entire healthcare system, after the default EMR prescription was changed from 30 to 12 tablets (24). The study concluded that reducing the default number of opioid pills on the EMR prescription is a simple, effective, inexpensive and potentially scalable solution, to decrease excessive postoperative opioid prescribing (24).
Alternatives to opioids in perioperative pain relief
Opioids have no analgesic ceiling and a fast onset of action which makes them effective analgesics in the perioperative period (1). However, consideration should be made to their harmful side effects, not just with respect to dependence and misuse. Common adverse effects include sedation, nausea, vomiting, respiratory depression, confusion, ileus and urinary retention; all barriers to postoperative recovery (1,15). They have also been associated with immunosuppression and endocrinopathy and more recent evidence has linked opioids to cancer (15,25).
The phenomenon of opioid-induced hyperalgesia secondary to nociceptive sensitisation following opioid administration, along with progressive opioid tolerance, can quickly result in the need for escalating doses. As such, minimising intraoperative doses of opioids is required to help reduce higher opioid requirements postoperatively (1). The growing list of adverse features means it is imperative to find analgesic alternatives in the perioperative period. Multimodal analgesia is now a widely adopted methodology in order to reduce the perioperative use of opioids and improve postoperative pain management. The use of multiple medications has a synergistic effect and is accepted as preferable to using opioids as a mono-therapy. The potential to incorporate multimodal analgesia occurs at all stages of the perioperative period.
Preoperatively, administration of analgesic medication immediately prior to an operation may have a beneficial effect on reducing perioperative opioid use. Both paracetamol and cyclooxygenase-2 (COX-2) inhibitors have been shown to reduce postoperative pain, nausea, vomiting and opioid use. While concern may lie over increased bleeding risk with COX-2 inhibitors, a meta-analysis showed no increase in bleeding in the intraoperative and postoperative periods with their use (26). Gabapentin may also help in this respect, however there is apprehension over associated side effects (namely sedation, dizziness and visual disturbance), as well as potential for long-term abuse (1,5).
From the intraoperative through to the postoperative period, the use of regional and neuraxial techniques are well known to reduce the use of perioperative opioids and form an essential component of any multimodal or ERAS pathway (1). While they enable opioid-free anaesthesia, even incorporation of opioids within them (e.g., intrathecal morphine or diamorphine) has been shown to reduce overall opioid consumption (5). These techniques can be used as a “one-shot block”, or preferably involve indwelling catheter placement to facilitate continuous administration of local anaesthetic in the days immediately following surgery (5).
More recently there has been additional focus on truncal fascial plane blocks for a variety of abdominal procedures (1). Not only have these been shown to reduce postoperative pain scores, but they may help functional status during the recovery period and reduce postoperative pulmonary complications (27). Even in those cases where regional anaesthesia is not appropriate, infiltration of local anaesthetic at the wound site can be useful in early postoperative pain management (5). In addition, intraoperative administration of analgesic adjuvants such as ketamine, magnesium, dexamethasone, non-steroidal anti-inflammatories (NSAIDS) and intravenous lidocaine have all been shown to be valuable in reducing postoperative opioid use (1,5). Many of these methods can be continued in the post-operative period, all with opioid-sparing effects.
Conclusions
Whilst opioids necessarily play a fundamental role in postoperative analgesic regimes, the opioid crisis is worsening from all perspectives: over-prescription, dependence, diversion and fatalities, exacerbated by a failure to universally define PPOU. The role of the perioperative healthcare team is essential in carefully planning, managing and reacting to potential unintended consequences, as well as preventing PPOU through meticulous prescribing habits.
This challenge is poised to get significantly harder, set against the backdrop of the COVID-19 pandemic, with extensive delays to elective pain-relieving surgery for patients across the world. Implementation of more novel scoring systems including functional assessments of pain and activity levels, alongside ERAS programs, multimodal analgesia and other innovative approaches to managing opioid prescription and dispensary techniques, will ease the burden.
Ultimately, identifying those at higher risk of PPOU, together with managing patient expectations around perioperative pain through clear communication, preparation and education, is vital to navigating these challenging waters and maintaining strong opioid stewardship.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Digestive Medicine Research for the series “Current Issues in Analgesia for Major Surgery”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-82/coif). The series “Current Issues in Analgesia for Major Surgery” was commissioned by the editorial office without any funding or sponsorship. CJ served as the unpaid Guest Editor of the series and serves as an unpaid Associate Editor-in-Chief of Digestive Medicine Research. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Koepke EJ, Manning EL, Miller TE, et al. The rising tide of opioid use and abuse: the role of the anesthesiologist. Perioper Med (Lond) 2018;7:16. [Crossref] [PubMed]
- Levy N, Sturgess J, Mills P. "Pain as the fifth vital sign" and dependence on the "numerical pain scale" is being abandoned in the US: Why? Br J Anaesth 2018;120:435-8. [Crossref] [PubMed]
- Kent ML, Hurley RW, Oderda GM, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative-4 Joint Consensus Statement on Persistent Postoperative Opioid Use: Definition, Incidence, Risk Factors, and Health Care System Initiatives. Anesth Analg 2019;129:543-52. [Crossref] [PubMed]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736-88. [Crossref] [PubMed]
- Soffin EM, Lee BH, Kumar KK, et al. The prescription opioid crisis: role of the anaesthesiologist in reducing opioid use and misuse. Br J Anaesth 2019;122:e198-208. [Crossref] [PubMed]
- Ayoo K, Mikhaeil J, Huang A, et al. The opioid crisis in North America: facts and future lessons for Europe. Anaesthesiol Intensive Ther 2020;52:139-47. [Crossref] [PubMed]
- Jivraj NK, Raghavji F, Bethell J, et al. Persistent Postoperative Opioid Use: A Systematic Literature Search of Definitions and Population-based Cohort Study. Anesthesiology 2020;132:1528-39. [Crossref] [PubMed]
- Higgins C, Smith BH, Matthews K. Incidence of iatrogenic opioid dependence or abuse in patients with pain who were exposed to opioid analgesic therapy: a systematic review and meta-analysis. Br J Anaesth 2018;120:1335-44. [Crossref] [PubMed]
- Gerrish AW, Fogel S, Lockhart ER, et al. Opioid prescribing practices during implementation of an enhanced recovery program at a tertiary care hospital. Surgery 2018;164:674-9. [Crossref] [PubMed]
- Levy N, Lobo DN, Fawcett WJ, et al. Opioid stewardship: a need for opioid discharge guidance. Comment on Br J Anaesth 2019; 122: e198-e208. Br J Anaesth 2019;122:e215-6. [Crossref] [PubMed]
- Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ 2018;360:j5790. [Crossref] [PubMed]
- Fiore JF Jr, Olleik G, El-Kefraoui C, et al. Preventing opioid prescription after major surgery: a scoping review of opioid-free analgesia. Br J Anaesth 2019;123:627-36. [Crossref] [PubMed]
- Marks JH. Lessons from Corporate Influence in the Opioid Epidemic: Toward a Norm of Separation. J Bioeth Inq 2020;17:173-89. [Crossref] [PubMed]
- Dyer O. WHO drops opioid guidelines after criticism of corporate influence. BMJ 2019;365:l4374. [Crossref] [PubMed]
- Hah JM, Bateman BT, Ratliff J, et al. Chronic Opioid Use After Surgery: Implications for Perioperative Management in the Face of the Opioid Epidemic. Anesth Analg 2017;125:1733-40. [Crossref] [PubMed]
- Zhao S, Chen F, Feng A, et al. Risk Factors and Prevention Strategies for Postoperative Opioid Abuse. Pain Res Manag 2019;2019:7490801. [Crossref] [PubMed]
- Shah A, Hayes CJ, Martin BC. Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use - United States, 2006-2015. MMWR Morb Mortal Wkly Rep 2017;66:265-9. [Crossref] [PubMed]
- Stone AB, Wick EC, Wu CL, et al. The US Opioid Crisis: A Role for Enhanced Recovery After Surgery. Anesth Analg 2017;125:1803-5. [Crossref] [PubMed]
- Compton WM, Jones CM, Baldwin GT. Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. N Engl J Med 2016;374:154-63. [Crossref] [PubMed]
- Lipari RH, Hughes MS. How People Obtain the Prescription Pain Relievers They Misuse. Substance Abuse and Mental Health Services Administration (US). 2017. Available online: https://www.samhsa.gov/data/sites/default/files/report_2686/ShortReport-2686.pdf
- Chebrolu G, O’Neill C, Albanawi Y. Pilleve. Interviewed by Dr Rachael Grimaldi, August 2021. Product Available on: https://www.pilleve.com/ [Accessed 01/10/21]
- Allan LD, Coyne C, Byrnes CM, et al. Tackling the opioid epidemic: Reducing opioid prescribing while maintaining patient satisfaction with pain management after outpatient surgery. Am J Surg 2020;220:1108-14. [Crossref] [PubMed]
- Brandal D, Keller MS, Lee C, et al. Impact of Enhanced Recovery After Surgery and Opioid-Free Anesthesia on Opioid Prescriptions at Discharge From the Hospital: A Historical-Prospective Study. Anesth Analg 2017;125:1784-92. [Crossref] [PubMed]
- Chiu AS, Jean RA, Hoag JR, et al. Association of Lowering Default Pill Counts in Electronic Medical Record Systems With Postoperative Opioid Prescribing. JAMA Surg 2018;153:1012-9. [Crossref] [PubMed]
- Heaney A, Buggy DJ. Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis? Br J Anaesth 2012;109:i17-28. [Crossref] [PubMed]
- Teerawattananon C, Tantayakom P, Suwanawiboon B, et al. Risk of perioperative bleeding related to highly selective cyclooxygenase-2 inhibitors: A systematic review and meta-analysis. Semin Arthritis Rheum 2017;46:520-8. [Crossref] [PubMed]
- Levy G, Cordes MA, Farivar AS, et al. Transversus Abdominis Plane Block Improves Perioperative Outcome After Esophagectomy Versus Epidural. Ann Thorac Surg 2018;105:406-12. [Crossref] [PubMed]
Cite this article as: Grimaldi R, Cassels-Barker A, Maskell N, Jones C. A painful pandemic: review of the opioid crisis and major surgery. Dig Med Res 2022;5:28.


