Malignant peritoneal mesothelioma literature review: past, present, and future
Introduction
Malignant peritoneal mesothelioma (MPM) is a rare, aggressive, and lethal neoplasm that arises from the mesothelial lining of the abdominal cavity (1-3). MPM comprises 20–30% of all mesothelioma in developed countries (4-6). Annually, approximately 500–800 new cases of MPM are diagnosed in the United States, with 90% diagnosed in non-Hispanic whites (7-10). Unlike pleural mesothelioma (PM), patients with peritoneal disease are diagnosed at a younger age (median 63 versus 71 years), with equal disease distribution between men and women (8). At the time of diagnosis, the majority of patients will have advanced disease; without treatment, median overall survival is at best 6 months (8,9,11). Cytoreductive surgery (CRS) followed by hyperthermic intraperitoneal chemotherapy (HIPEC) is the standard of care for patients with resectable disease and good performance status (8,12,13). With advances in operative technique, CRS-HIPEC has shown encouraging five-year survival results, and in some settings this is performed with intention to cure. On the other hand, outcomes for unresectable MPM remains dismal, with median survival of 1 year (14). This review provides an overview of our current knowledge concerning MPM including epidemiology, clinical presentation, and diagnosis; as well as discussions of past, present, and future directions of treatment. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-19/rc).
Methods
A comprehensive literature review was completed by searching PubMed, Google Scholar, and ClinicalTrials.gov from database’s inception until January 31, 2022. Only articles published in English were considered. All natures of studies: prospective randomized controlled trials, non-randomized prospective trials, retrospective studies, case reports, reviews, and meta-analyses were included. The search terms included singular and combinations of the following: “malignant peritoneal mesothelioma”, “mesothelioma”, “cytoreductive surgery”, “heated intraperitoneal chemotherapy”, and “peritoneal carcinomatosis” (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | January 31, 2022 |
| Databases and other sources searched | PubMed, Google Scholar, and ClinicalTrials.gov |
| Search terms used | Search terms: malignant peritoneal mesothelioma, mesothelioma, cytoreductive surgery, heated intraperitoneal chemotherapy, and peritoneal carcinomatosis |
| Timeframe | From origin until January 31, 2022 |
| Inclusion and exclusion criteria | Inclusion and exclusion criteria: (I) articles in English languages; (II) article types: prospective randomized controlled trials, non-randomized prospective trials, retrospective studies, case reports, reviews, and meta-analyses |
| Selection process | Selection was conducted by all authors |
Epidemiology
MPM was first described in 1908 by Miller and Wynn; however, it gained attention during the 1960s after the effects of widespread use of asbestos during World War II were shown to correlate with increased incidence rates of mesothelioma and disease-specific mortality (2). In the 1980s, commercial regulations were implemented to ban the use of six mineral fibers collectively defined as “asbestos”. However, many of the remaining 400 mineral fibers are unregulated; they are still commercially used today, considered carcinogenic, and have been associated with mesothelioma (15). In fact, talc-based products have been associated with mesothelioma and ovarian cancer due to containing small amounts of asbestos minerals such as chrysotile and amphiboles. Recently, Johnson & Johnson withdrew its sale of talc baby powder in the United States and Canada due to lawsuits claiming association with ovarian cancer and mesothelioma (16). Today, asbestos remains the most identifiable risk factor in MPM; however, only 10–33% of MPM cases have positively identified previous asbestos exposure. MPM takes 20 years to develop following exposure, thus recall to the exposure can be difficult (9,15,17). Talcum, historic Thorotrast angiograph dye, radiation, papovavirus, simian virus, and chronic inflammation represent additional risk factors for the development of MPM (17,18).
Clinical features
Patients with MPM most commonly present with vague symptoms such as nonspecific abdominal pain or bloating. Patients may develop abdominal distention secondary to ascites or a palpable abdominal mass (8). Patients can also exhibit signs of extensive abdominal disease such as early satiety, weight loss, and fatigue (11). Time of symptom onset to diagnosis is approximately 4–6 months, but frequently longer given the vague nature of initial associated symptoms (19).
Diagnosis
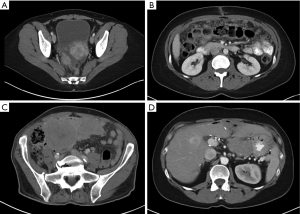
Computed tomography (CT) is the principal imaging modality utilized for diagnosis. While positron emission tomography (PET) can be used in conjunction with CT, its value in initial diagnosis is not clear (20). Radiographically, the disease may present as mesenteric or parietal peritoneal nodules, visceral peritoneal thickening leading to foreshortening of the mesentery, ascites, or omental masses (21). Favorable radiographic findings include minimal soft tissue masses, normal intestine and mesenteric anatomy, and lack of ascites. Unfavorable findings include tumors >5 cm, especially tumors involving the lesser omentum or jejunal regions; para-aortic lymphadenopathy, and diffuse peritoneal thickening resulting in anatomic distortion of the bowel (8,22,23). A hallmark feature of MPM is its propensity to remain confined to the abdominal cavity. In fact, extra-abdominal disease is rare and typically only manifests by direct disease extension from the diaphragm across into the pleural space or via extra-abdominal lymph node metastasis (11,24) (Figure 1).
The serum protein cancer antigen (CA)-125 is frequently elevated in patients with MPM and used as a tumor marker. Due to the higher prevalence of ovarian cancer and its established association with elevated CA-125, approximately one-third of women with MPM are initially misdiagnosed with ovarian cancer (16,25). While the low specificity of CA-125 limits its utility in diagnosing MPM, it has shown some use for tumor progression and surveillance since CA-125 levels often normalize after treatment (25).
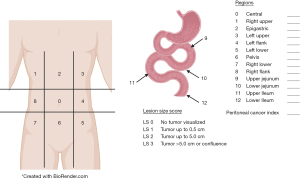
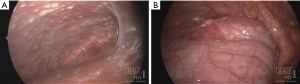
In conjunction with imaging for diagnosis, diagnostic laparoscopy has the added advantage of direct visualization of peritoneal disease burden and ability to obtain tumor biopsies (Figure 2). Diagnostic laparoscopy is imperative for staging a patient’s tumor burden and identifying patients whose disease is amenable to complete cytoreduction, particularly those patients without overt radiographic evidence of disease. The peritoneal cancer index (PCI) is a systematic method of determining the distribution and burden of disease for staging (26). The PCI is calculated by dividing the peritoneal cavity into 13 regions. The peritoneal cavity is then further subdivided into 9 regions with numbering starting at the right hemidiaphragm and moving in a clockwise fashion (the umbilical region is labeled 0), followed by 4 regions of the small bowel (proximal and distal jejunum and ileum) (Figure 3). Laparoscopy, however, will underestimate to some degree the extent of disease (8). Laparoscopic port sites can also be sources for tumor deposit, so it is important to limit the number of port sites and place them in the linea alba to be excised during CRS-HIPEC if possible.
Pathology
MPM is composed of three histological subtypes: epithelioid (56%), sarcomatoid (31%), and mixed/biphasic type (13%) (1,11). Epithelioid MPM is considered the least aggressive subtype and is associated with the most favorable prognosis in general (11,27). Borderline malignant variants of peritoneal mesothelioma have also been described, such as benign multicystic mesothelioma and well-differentiated papillary mesothelioma (WDPM). These variants are considered different biological entities and can usually be differentiated from their malignant counterparts through lack of invasion as well as no increased cellularity within the stroma or simple papillary formations if present (28,29). Of note, there is controversy in the literature regarding whether WDPM is a neoplasm or a reactive process, and there have been some case reports of WDPM with invasive foci (30,31) (Figure 4).
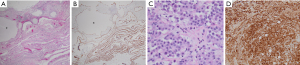
Once biopsies are obtained, a definite diagnosis of mesothelioma can be confirmed using a panel of immunohistochemical (IHC) antibodies. The most sensitive IHC markers for mesothelioma include calretinin, cytokeratin 5/6, and Wilm’s tumor (WT-1) (8,11). IHC markers mesothelin and fibulin-3 are specific for mesothelioma (32). Negative antibody staining for carcinoembryonic antigen (CEA) and PAX8 are imperative to distinguish pathologic diagnosis of MPM from another primary such as colon cancer or ovarian cancer (17,33). IHC can also be used to look for loss of BAP1 expression. BRCA-associated protein 1 (BAP1) is involved in DNA repair and apoptosis of DNA mutations (34). BAP1 mutation is associated with uveal melanoma, clear cell renal carcinoma, cutaneous malignancies, and mesothelioma (15,35). BAP1 mutation has been detected in 27–67% of PMs (15,36). There are few studies examining prevalence of BAP1 mutation in peritoneal mesothelioma, but one study by Singhi et al. revealed a loss of BAP1 in 57% of their patients (n=49) (36).
In fact, many molecular alterations for mesothelioma such as NF2, CDK2A/B, TP53, and SETD2 have been reported in the literature for PM, but a paucity of genomic data exists for MPM (37). A recent series from Memorial Sloan Kettering used next-generation sequencing on fifty MPM tumors and revealed alterations in BAP1 (60%), NF2 (24%), SETD2 (22%), and TP53 (16%) (37). Regarding BAP1 alterations in this study, overall survival was worse when mutations or deletions on next-generation sequencing coexisted with loss on IHC as to compared wild-type BAP1 or retained expression on IHC (37). In contrast, BAP1 mutation and loss of expression has been associated with improved survival in PM, and a study in France reported better overall survival for MPM independent of tumor histological subtype, age, and sex (38,39).
Treatment
Historically, treatment of MPM remained palliative in intent; however, systemic chemotherapy was introduced as an early alternative after the management of six patients by Brenner et al. (40). Decades later, the combination of surgical resection and regional intraperitoneal chemotherapy became more widespread. The first multi-institutional consensus meeting was hosted by the National Cancer Institute in Bethesda, Maryland in 2004, and the second international consensus from the Peritoneal Surface Oncology Group Biennial Meeting was held in Milan, Italy in 2006 (20,22,41). Together, these meetings resulted in the new standard of care for MPM: CRS combined with HIPEC.
Surgery
The introduction of CRS in combination with HIPEC has improved the prognosis of selected patients with MPM to a median survival of 30–100 months and an overall 5-year survival of 40–70% (22,42). The goal for therapeutic treatment is complete or near-complete removal of all visible disease, as indicated by the Completeness of Cytoreduction (CC) score of 0 or 1 (43). In order to achieve complete cytoreduction, resections are comprised of parietal peritonectomy, selective visceral peritonectomy, greater and lesser omentectomy, and cholecystectomy. Organ resections may include splenectomy, enterectomy, or partial colectomy including low anterior resection en bloc with pelvic peritonectomy. Given the frequently extensive involvement of the diaphragmatic peritoneum, partial stripping of the hepatic capsule may be required. Some controversy does exist regarding selective peritonectomy of only grossly involved peritoneum versus routine complete parietal peritonectomy. One retrospective study reported a 5-year survival of 40% with selective peritonectomy, and 63.9% with complete parietal peritonectomy (P=0.027) (22,44). However, prospective randomized data are not available. Furthermore, the role of routine complete parietal peritonectomy in the setting of germline BAP1 mutation remains undefined.
HIPEC
Following CRS, HIPEC is preformed to address the remaining microscopic disease and residual tumor deposits <2.5 mm in size (8). Intraperitoneal chemotherapy is heated to a temperature of approximately 42 degrees Celsius. The hyperthermia acts synergistically with the cytotoxic intraperitoneal chemotherapy to facilitate drug uptake as well as to denature proteins within susceptible malignant cells and thereby induce heat-shock proteins (HSP), inhibit angiogenesis, promote apoptosis, and impair DNA repair (8,45). Intraperitoneal chemotherapy can only penetrate up to 2–3 mm deep, thus reinforcing the importance of a complete cytoreduction. In cases where complete CRS cannot be obtained, HIPEC may be considered for palliation, especially in patients with ascites (22).
There are two main methods for delivering intraperitoneal chemotherapy: the “open” (or “coliseum”) technique and the “closed” technique. The “open” technique involves delivering chemotherapy to an open peritoneal cavity. This is typically achieved by suturing the skin to a overlying ring retractor above the wound, which allows the surgeon’s hand to be introduced directly into the abdomen to allow for manual stirring and allocation of the peritoneal contents during HIPEC (45). The “closed” technique uses a running skin suture to bring together the laparotomy incision around the perfusing catheters to temporarily close the abdomen. Although the “closed” technique allows less exposure to operating room staff, there have been no studies directly comparing the two techniques in humans. Thus, technique is currently based on surgeon preference.
Despite its use for decades, intraperitoneal chemotherapy has not yet been standardized. Cisplatin and Mitomycin C (MMC) are most commonly reported in the literature as intraperitoneal agents used for MPM. Cisplatin is an alkylating agent that forms DNA adducts leading to apoptosis and has long been studied in its use for intraperitoneal administration (46). MMC is an alkylating agent that cross-links DNA and is recognized as one of the earliest HIPEC agents used in clinical trials of CRS-HIPEC (47). The 2018 RENAPE study was a retrospective study from France comparing the various HIPEC agents with survival outcomes after CRS-HIPEC (42). This study compared 249 patients with MPM who received either cisplatin, cisplatin plus doxorubicin, MMC, oxaliplatin, or oxaliplatin with irinotecan. No statistical differences were found among the different chemotherapy agents; however, overall survival (OS) was better in patients who received combined chemotherapy compared to a single agent [hazard ratio (HR) 0.54, 95% CI: 0.31–0.95; P=0.03] (42). In particular, patients with CC-0 resections and epithelioid histological subtype had better OS and PFS with dual-agent chemotherapy compared to single-agent treatment and had no increased postoperative morbidity (42). Another study with 211 patients with MPM observed greater overall survival with cisplatin compared to MMC in patients with CC0 or CC1 (P<0.02); there was no difference in cisplatin versus MMC for patients with CC2 disease (48). A smaller study out of Wake Forest University also published a trend towards improved survival with cisplatin versus MMC (49).
Over the past 20 years, Sugarbaker has produced results for three phase II treatment protocols for patients with MPM. In the first protocol, 42 patients were administered CRS-HIPEC with doxorubicin and cisplatin. The second protocol examined 58 patients who were treated with CRS-HIPEC, but were then followed by early postoperative intraperitoneal chemotherapy (EPIC) with daily paclitaxel for 5 days following CRS. The third protocol delivered CRS-HIPEC, EPIC, and then long-term intraperitoneal paclitaxel or pemetrexed plus IV cisplatin as adjuvant normothermic intraperitoneal chemotherapy (NIPEC) to 29 patients (50). Long-term regional chemotherapy was associated with improved survival outcomes, with 5-year overall survival of 44%, 52%, and 75% respectively for the above protocols. While no significant difference was demonstrated by the addition of EPIC to CRS-HIPEC, there was a statistically significant increase in survival among patients given NIPEC (P=0.037) (50). Despite encouraging results, the cohorts above are small, limited to a single institution, and are non-randomized. Further phase II trials and multi-institutional protocols are needed to better demonstrate the potential additional therapeutic value of NIPEC compared to HIPEC and/or EPIC alone.
More recently, pressurized intraperitoneal aerosol chemotherapy (PIPAC) has been proposed as a new technique to deliver intraperitoneal chemotherapy. Data from preclinical studies and a small three-patient study demonstrated higher local drug bioavailability and better therapeutic index after PIPAC (doxorubicin and cisplatin) versus HIPEC (51). A prospective study in France looked at the use of PIPAC in small cohort of patients with diffuse MPM (52). The purpose of this study was to determine feasibility of PIPAC as neoadjuvant treatment with intent of facilitating future CRS. Of 25 patients reviewed, 20 were considered nonresectable upfront and administered IV chemotherapy along with cisplatin and doxorubicin as PIPAC. Of the 20 patients, 11 patients underwent complete CRS after receiving PIPAC (52). Another study out of France evaluated neoadjuvant catheter-based bidirectional treatment in patients with diffuse MPM initially considered unresectable or borderline resectable. Twenty patients were given neoadjuvant IP pemetrexed with IV cisplatin or IP oxaliplatin with IV gemcitabine in effort to facilitate future CRS-HIPEC. After post-treatment laparoscopic reevaluation, 50% of the patients were able to undergo complete CRS-HIPEC. The two-year overall survival was 83.3% for patients who were able to achieve CRS-HIPEC and 44% for patients treated with bidirectional therapy only (53). These novel intraperitoneal chemotherapy approaches to otherwise high disease burdens offers the potential for downstaging MPM and facilitating surgical treatment.
Chemotherapy
MPM renders many patients unresectable at diagnosis. The efficacy of systemic chemotherapy is poor as MPM is associated with relative chemoresistance (20). Cisplatin or carboplatin has shown some benefit as monotherapy and in combination with gemcitabine (20,54). A phase III clinical trial comparing pemetrexed with cisplatin compared to cisplatin alone showed increased response rate and median overall survival of 9.3 vs. 12.1 months in patients with PM who received combination therapy (55). Pemetrexed was well tolerated, with low rates of grade 3 or 4 side effects (56). Following this trial, the International Expanded Access Program (EAP) was created to facilitate compassionate use of pemetrexed for 109 patients with MPM. Response rates were higher when pemetrexed was combined with a platinum agent versus pemetrexed alone, and disease control rate was 78% in combination versus 50% alone (56). A phase II clinical trial revealed that the treatment with pemetrexed and gemcitabine had inferior survival results (1 year OS 67.5%), and toxicity was significant (11,57). Additionally, bevacizumab, a monoclonal antibody (mAb) against VEGFA, has been shown to be associated with statistically significant overall survival (P=0.017) when combined with cisplatin and pemetrexed in treatment of PM (58). A multicenter phase II study investigated the use of this combination in 53 patients with advanced malignant mesothelioma, including a small cohort of MPM (n=7), however this paper largely focused on PM and the trial failed to meet the primary endpoint of 33% improvement in progression-free survival rate at 6 months compared to controls with cisplatin and pemetrexed alone (59). Further trials with the addition of bevacizumab to cisplatin and pemetrexed are needed in regards to treatment of MPM. At this point, pemetrexed with cisplatin is considered standard first line systemic treatment for patients with unresectable disease, whereas pemetrexed and gemcitabine can be used as a second line therapy for patients who cannot tolerate platinum therapy (57).
Immunotherapy
Of late, immune checkpoint inhibitors (ICI) have been an area of interest for second-line treatment of patients with MPM who have progressed on or were intolerant to prior first-line platinum-pemetrexed chemotherapy (60). ICIs have shown efficacy in MPM and have recently been approved for first-line treatment; however, the evidence for efficacy of ICI in MPM has been limited. PD-L1 expression is observed in approximately 50% of patients with MPM compared to 30% with PM (61). In a phase II trial accruing both PM and MPM patients, pembrolizumab had an overall response rate (ORR) of 20% in pleural (n=56) versus 12.5% in peritoneal (n=8) mesothelioma (60). A second phase II trial investigated the use of “AtezoBev” (Atezolizumab, mAb against PD-L1, with bevacizumab) in 20 patients with advanced yet previously treated MPM. AtezoBev showed a promising 40% ORR with notable 1 year progression free survival (PFS) of 61% and 1 year OS of 85% (60,62). Another clinical trial looked at a total of 29 patients with MPM, with 20 treated with dual ICIs (nivolumab plus ipilimumab) and 9 treated with single agent ICI (63). This trial found no significant difference in overall response rate (ORR) when comparing dual agent with single agent ICIs (63). Head-to-head comparisons of single- or dual-agent ICI therapy versus second-line cytotoxic chemotherapy remain to be performed.
Future directions
Currently, there are several ongoing and upcoming clinical trials focusing on the treatment of MPM. For instance, a phase II randomized clinical trial, NCT05001880, has been accepted to compare the addition of atezolizumab with carboplatin, pemetrexed, and bevacizumab prior to surgery compared to a control group without the addition of atezolizumab treatment. Another upcoming clinical trial, NCT05041062, is focusing on major pathologic response of peritoneal mesothelioma tumors to the preoperative combination of nivolumab and ipilimumab. An active clinical trial, NCT04847068, is utilizing an ex-vivo SMART (Sample Microenvironment of Resected Metastatic Tumor) System to test tumor response to different intraperitoneal chemotherapy agents in patients with peritoneal carcinomatosis, including peritoneal mesothelioma.
Chimeric antigen receptor (CAR)-expressing T cells represent another avenue of immunotherapy that shows potential in mesothelioma. A phase I clinical trial, NCT01583686, used CAR T cell receptor immunology targeting mesothelin for patients with metastatic disease. Mesothelin is a tumor differentiation antigen that is overexpressed in many malignancies including ovarian cancer and mesothelioma (64). Hassan et al., investigated MORAb-009, a chimerical monoclonal antibody targeting mesothelin in patients with mesothelioma (n=13), pancreatic cancer (n=7), and ovarian cancer (n=4). This phase I study revealed MORAb-009 to be well tolerated with maximum tolerated dose (MTD) of 200 mg/m2, and 11 of 24 patients treated had stable disease (64). A phase II study was implemented, however the malignancies targeted were pancreatic cancer, ovarian cancer, and PM. Hassan et al., also utilized SS1P, a recombinant mesothelin immunotoxin consisting of the anti-mesothelin Fv linked to a truncated form of the potent bacterial toxin Pseudomonas exotoxin A, for treatment in patients with malignant mesothelioma and other mesothelin-expressing cancers (65). A phase I clinical trial established safety and maximum MTD of SS1P, which led to a pilot trial of SS1P in combination with pemetrexed and cisplatin in chemo-naive patients with PM that resulted in 8 out of 13 patients having partial responses. Currently, SS1P is being combined with Pentostatin plus cyclophosphamide in clinical trial NCT0136290 to study the effectiveness of suppressing the immune system in chemo-refractory malignant mesothelioma. More recently, a cell surface antigen, mesothelin Immunotoxin LMB-100, a recombinant anti-mesothelin immunotoxin composed of humanized anti-mesothelin Fab fused to a Pseudomonas exotoxin, has been studied in a phase I trial in patients with advanced pleural or peritoneal mesothelioma who did not respond to platinum therapy (66). Ten patients were treated with LMB-100 and then treated with either pembrolizumab (n=9) or nivolumab (n=1). Four of the ten patients had either partial response or complete response. Due to promising results, a phase II clinical trial, NCT03644550, is currently recruiting patients with pleural and peritoneal mesothelioma who have progressed on platinum therapy to be treated with LMB100 followed by pembrolizumab (67). While the results are not currently available, the variation of clinical trials and advancing technology elicits hope for future treatments.
Conclusions
MPM is a rare and lethal disease of the peritoneal lining. CRS-HIPEC remains the standard of care and is associated with long-term survival in patients who receive complete or near-complete cytoreduction. While not as effective, systemic therapy, immune checkpoint inhibitors, investigational agents, NIPEC, and PIPAC are available for patients with advanced nonresectable disease.
Acknowledgments
Funding: This study was funded by the National Institute of Health, Intramural Research Program.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Digestive Medicine Research, for the series “Peritoneal Carcinomatosis: History and Future”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-19/rc
Peer Review File: Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-19/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-19/coif). The series “Peritoneal Carcinomatosis: History and Future” was commissioned by the editorial office without any funding or sponsorship. AMB served as the unpaid Guest Editor of the series. The authors are funded by the National Institute of Health, Intramural Research Program. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mirarabshahii P, Pillai K, Chua TC, et al. Diffuse malignant peritoneal mesothelioma--an update on treatment. Cancer Treat Rev 2012;38:605-12. [Crossref] [PubMed]
- Bridda A, Padoan I, Mencarelli R, et al. Peritoneal mesothelioma: a review. MedGenMed 2007;9:32. [PubMed]
- Raptopoulos V. Peritoneal mesothelioma. Crit Rev Diagn Imaging 1985;24:293-328. [PubMed]
- Tanida S, Kataoka H, Kubota E, et al. Combination chemotherapy with cisplatin and gemcitabine in malignant peritoneal mesothelioma. Int J Clin Oncol 2009;14:266-9. [Crossref] [PubMed]
- Scripcariu V, Dajbog E, Radu I, et al. Malignant peritoneal mesothelioma tumours. Evolution, treatment, prognosis. Rev Med Chir Soc Med Nat Iasi 2007;111:673-7. [PubMed]
- Souza FF, Jagganathan J, Ramayia N, et al. Recurrent malignant peritoneal mesothelioma: radiological manifestations. Abdom Imaging 2010;35:315-21. [Crossref] [PubMed]
- Roife D, Powers BD, Zaidi MY, et al. CRS/HIPEC with Major Organ Resection in Peritoneal Mesothelioma Does not Impact Major Complications or Overall Survival: A Retrospective Cohort Study of the US HIPEC Collaborative. Ann Surg Oncol 2020;27:4996-5004. [Crossref] [PubMed]
- Greenbaum A, Alexander HR. Peritoneal mesothelioma. Transl Lung Cancer Res 2020;9:S120-32. [Crossref] [PubMed]
- Li CY, Alexander HR Jr. Peritoneal Metastases from Malignant Mesothelioma. Surg Oncol Clin N Am 2018;27:539-49. [Crossref] [PubMed]
- Miura JT, Johnston FM, Gamblin TC, et al. Current trends in the management of malignant peritoneal mesothelioma. Ann Surg Oncol 2014;21:3947-53. [Crossref] [PubMed]
- Alexander HR Jr, Burke AP. Diagnosis and management of patients with malignant peritoneal mesothelioma. J Gastrointest Oncol 2016;7:79-86. [PubMed]
- Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009;27:6237-42. [Crossref] [PubMed]
- Feldman AL, Libutti SK, Pingpank JF, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 2003;21:4560-7. [Crossref] [PubMed]
- Baratti D, Kusamura S, Cabras AD, et al. Diffuse malignant peritoneal mesothelioma: long-term survival with complete cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Cancer 2013;49:3140-8. [Crossref] [PubMed]
- Carbone M, Adusumilli PS, Alexander HR Jr, et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA Cancer J Clin 2019;69:402-29. [Crossref] [PubMed]
- Slomovitz B, de Haydu C, Taub M, et al. Asbestos and ovarian cancer: examining the historical evidence. Int J Gynecol Cancer 2021;31:122-8. [Crossref] [PubMed]
- Boussios S, Moschetta M, Karathanasi A, et al. Malignant peritoneal mesothelioma: clinical aspects, and therapeutic perspectives. Ann Gastroenterol 2018;31:659-69. [Crossref] [PubMed]
- Boffetta P. Epidemiology of peritoneal mesothelioma: a review. Ann Oncol 2007;18:985-90. [Crossref] [PubMed]
- Enomoto LM, Shen P, Levine EA, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal mesothelioma: patient selection and special considerations. Cancer Manag Res 2019;11:4231-41. [Crossref] [PubMed]
- Deraco M, Bartlett D, Kusamura S, et al. Consensus statement on peritoneal mesothelioma. J Surg Oncol 2008;98:268-72. [Crossref] [PubMed]
- Park JY, Kim KW, Kwon HJ, et al. Peritoneal mesotheliomas: clinicopathologic features, CT findings, and differential diagnosis. AJR Am J Roentgenol 2008;191:814-25. [Crossref] [PubMed]
- Sugarbaker PH. Update on the management of malignant peritoneal mesothelioma. Transl Lung Cancer Res 2018;7:599-608. [Crossref] [PubMed]
- Sugarbaker PH, Turaga KK, Alexander HR Jr, et al. Management of Malignant Peritoneal Mesothelioma Using Cytoreductive Surgery and Perioperative Chemotherapy. J Oncol Pract 2016;12:928-35. [Crossref] [PubMed]
- Magge D, Zenati MS, Austin F, et al. Malignant peritoneal mesothelioma: prognostic factors and oncologic outcome analysis. Ann Surg Oncol 2014;21:1159-65. [Crossref] [PubMed]
- Kim J, Bhagwandin S, Labow DM. Malignant peritoneal mesothelioma: a review. Ann Transl Med 2017;5:236. [Crossref] [PubMed]
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359-74. [Crossref] [PubMed]
- Cerruto CA, Brun EA, Chang D, et al. Prognostic significance of histomorphologic parameters in diffuse malignant peritoneal mesothelioma. Arch Pathol Lab Med 2006;130:1654-61. [Crossref] [PubMed]
- Daya D, McCaughey WT. Pathology of the peritoneum: a review of selected topics. Semin Diagn Pathol 1991;8:277-89. [PubMed]
- Myers DJ, Babiker HM. Benign Mesothelioma. StatPearls. Treasure Island (FL), 2021.
- Shrestha R, Nabavi N, Volik S, et al. Well-Differentiated Papillary Mesothelioma of the Peritoneum Is Genetically Distinct from Malignant Mesothelioma. Cancers (Basel) 2020;12:1568. [Crossref] [PubMed]
- Churg A, Allen T, Borczuk AC, et al. Well-differentiated papillary mesothelioma with invasive foci. Am J Surg Pathol 2014;38:990-8. [Crossref] [PubMed]
- Turaga KK, Deraco M, Alexander HR. Current management strategies for peritoneal mesothelioma. Int J Hyperthermia 2017;33:579-81. [Crossref] [PubMed]
- Cao S, Jin S, Cao J, et al. Advances in malignant peritoneal mesothelioma. Int J Colorectal Dis 2015;30:1-10. [Crossref] [PubMed]
- Baumann F, Flores E, Napolitano A, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis 2015;36:76-81. [Crossref] [PubMed]
- Kittaneh M, Berkelhammer C. Detecting germline BAP1 mutations in patients with peritoneal mesothelioma: benefits to patient and family members. J Transl Med 2018;16:194. [Crossref] [PubMed]
- Singhi AD, Krasinskas AM, Choudry HA, et al. The prognostic significance of BAP1, NF2, and CDKN2A in malignant peritoneal mesothelioma. Mod Pathol 2016;29:14-24. [Crossref] [PubMed]
- Offin M, Yang SR, Egger J, et al. Molecular Characterization of Peritoneal Mesotheliomas. J Thorac Oncol 2022;17:455-60. [Crossref] [PubMed]
- Carr NJ. New insights in the pathology of peritoneal surface malignancy. J Gastrointest Oncol 2021;12:S216-29. [Crossref] [PubMed]
- Leblay N, Leprêtre F, Le Stang N, et al. BAP1 Is Altered by Copy Number Loss, Mutation, and/or Loss of Protein Expression in More Than 70% of Malignant Peritoneal Mesotheliomas. J Thorac Oncol 2017;12:724-33. [Crossref] [PubMed]
- Brenner J, Sordillo PP, Magill GB, et al. Malignant peritoneal mesothelioma: review of 25 patients. Am J Gastroenterol 1981;75:311-3. [PubMed]
- Hassan R, Alexander R, Antman K, et al. Current treatment options and biology of peritoneal mesothelioma: meeting summary of the first NIH peritoneal mesothelioma conference. Ann Oncol 2006;17:1615-9. [Crossref] [PubMed]
- Malgras B, Gayat E, Aoun O, et al. Impact of Combination Chemotherapy in Peritoneal Mesothelioma Hyperthermic Intraperitoneal Chemotherapy (HIPEC): The RENAPE Study. Ann Surg Oncol 2018;25:3271-9. [Crossref] [PubMed]
- Sugarbaker PH. Management of peritoneal-surface malignancy: the surgeon's role. Langenbecks Arch Surg 1999;384:576-87. [Crossref] [PubMed]
- Baratti D, Kusamura S, Cabras AD, et al. Cytoreductive surgery with selective versus complete parietal peritonectomy followed by hyperthermic intraperitoneal chemotherapy in patients with diffuse malignant peritoneal mesothelioma: a controlled study. Ann Surg Oncol 2012;19:1416-24. [Crossref] [PubMed]
- Witkamp AJ, de Bree E, Van Goethem R, et al. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev 2001;27:365-74. [Crossref] [PubMed]
- Los G, Mutsaers PH, van der Vijgh WJ, et al. Direct diffusion of cis-diamminedichloroplatinum(II) in intraperitoneal rat tumors after intraperitoneal chemotherapy: a comparison with systemic chemotherapy. Cancer Res 1989;49:3380-4. [PubMed]
- Van der Speeten K, Lemoine L, Sugarbaker P. Overview of the optimal perioperative intraperitoneal chemotherapy regimens used in current clinical practice. Pleura Peritoneum 2017;2:63-72. [Crossref] [PubMed]
- Alexander HR Jr, Bartlett DL, Pingpank JF, et al. Treatment factors associated with long-term survival after cytoreductive surgery and regional chemotherapy for patients with malignant peritoneal mesothelioma. Surgery 2013;153:779-86. [Crossref] [PubMed]
- Blackham AU, Shen P, Stewart JH, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for malignant peritoneal mesothelioma: mitomycin versus cisplatin. Ann Surg Oncol 2010;17:2720-7. [Crossref] [PubMed]
- Sugarbaker PH, Chang D. Long-term regional chemotherapy for patients with epithelial malignant peritoneal mesothelioma results in improved survival. Eur J Surg Oncol 2017;43:1228-35. [Crossref] [PubMed]
- Solass W, Kerb R, Mürdter T, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 2014;21:553-9. [Crossref] [PubMed]
- Giger-Pabst U, Demtröder C, Falkenstein TA, et al. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) for the treatment of malignant mesothelioma. BMC Cancer 2018;18:442. [Crossref] [PubMed]
- Le Roy F, Gelli M, Hollebecque A, et al. Conversion to Complete Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Malignant Peritoneal Mesothelioma After Bidirectional Chemotherapy. Ann Surg Oncol 2017;24:3640-6. [Crossref] [PubMed]
- Kitadai R, Shimoi T, Sudo K, et al. Efficacy of second-line treatment and prognostic factors in patients with advanced malignant peritoneal mesothelioma: a retrospective study. BMC Cancer 2021;21:294. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Carteni G, Manegold C, Garcia GM, et al. Malignant peritoneal mesothelioma-Results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer 2009;64:211-8. [Crossref] [PubMed]
- Simon GR, Verschraegen CF, Jänne PA, et al. Pemetrexed plus gemcitabine as first-line chemotherapy for patients with peritoneal mesothelioma: final report of a phase II trial. J Clin Oncol 2008;26:3567-72. [Crossref] [PubMed]
- Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14. [Crossref] [PubMed]
- Dowell JE, Dunphy FR, Taub RN, et al. A multicenter phase II study of cisplatin, pemetrexed, and bevacizumab in patients with advanced malignant mesothelioma. Lung Cancer 2012;77:567-71. [Crossref] [PubMed]
- Raghav K, Liu S, Overman MJ, et al. Efficacy, Safety, and Biomarker Analysis of Combined PD-L1 (Atezolizumab) and VEGF (Bevacizumab) Blockade in Advanced Malignant Peritoneal Mesothelioma. Cancer Discov 2021;11:2738-47. [Crossref] [PubMed]
- Chapel DB, Stewart R, Furtado LV, et al. Tumor PD-L1 expression in malignant pleural and peritoneal mesothelioma by Dako PD-L1 22C3 pharmDx and Dako PD-L1 28-8 pharmDx assays. Hum Pathol 2019;87:11-7. [Crossref] [PubMed]
- Bijelic L, Darcy K, Stodghill J, et al. Predictors and Outcomes of Surgery in Peritoneal Mesothelioma: an Analysis of 2000 Patients from the National Cancer Database. Ann Surg Oncol 2020;27:2974-82. [Crossref] [PubMed]
- Raghav K, Liu S, Overman M, et al. Clinical Efficacy of Immune Checkpoint Inhibitors in Patients With Advanced Malignant Peritoneal Mesothelioma. JAMA Netw Open 2021;4:e2119934. [Crossref] [PubMed]
- Hassan R, Cohen SJ, Phillips M, et al. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res 2010;16:6132-8. [Crossref] [PubMed]
- Hassan R, Sharon E, Thomas A, et al. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 2014;120:3311-9. [Crossref] [PubMed]
- Hassan R, Alewine C, Mian I, et al. Phase 1 study of the immunotoxin LMB-100 in patients with mesothelioma and other solid tumors expressing mesothelin. Cancer 2020;126:4936-47. [Crossref] [PubMed]
- Hu ZI, Ghafoor A, Sengupta M, et al. Malignant mesothelioma: Advances in immune checkpoint inhibitor and mesothelin-targeted therapies. Cancer 2021;127:1010-20. [Crossref] [PubMed]
Cite this article as: Gregory SN, Sarvestani AL, Blakely AM. Malignant peritoneal mesothelioma literature review: past, present, and future. Dig Med Res 2022;5:29.