
Ultrasound-guided parenchima-sparing liver surgery: is it feasible by laparoscopy? Personal experience and narrative review of the literature
Introduction
Liver resection (LR) remains the gold standard of choice for curative treatment of both primary and secondary liver tumors. Much progress has been made particularly in the treatment of colorectal liver metastases (CLM), also thanks to the improvement of systemic therapies in the recent years (1-3).
Surgical strategy should aim to achieve oncological radicality while preserving enough liver parenchyma (4). Given these mainstreams, the future liver remnant (FLR) has represented a major limitation for expanding resectability particularly in case of multiple CLM. The two stage hepatectomy (TSH) (4,5) and ALPPS (associated liver partition and portal vein ligation for staged hepatectomy) (6) have been proposed to maximize liver regeneration and allow for major hepatectomies in these cases. The not negligible drop out of TSH and the relatively high mortality and morbidity of ALPPS (7) are the principal limits of these approaches.
In early 2000 the so-called radical but conservative policy was introduced based on the guidance of intraoperative ultrasound (IOUS) (8-10). This strategy aimed to challenge deeply located lesions in a conservative way with the aim of sparing the vascular-biliary architecture of the liver. The encouraging preliminary results (11), more recently confirmed on larger series (12,13) showing a similar risk of relapse between R0 resections and detachment of tumors from the vessels, known as R1vasc surgery, reinforced the effectiveness of the parenchymal sparing philosophy. Laparoscopic resective surgery has been shown to be safe and effective in the treatment of CLM, however the real feasibility of laparoscopic parenchyma-sparing hepatectomies (l-PSH) is still a matter of debate: mostly small series with a limited number of patients have been up to now published, mainly because of the technical difficulty of the procedure making the indication to the laparoscopic approach infrequent in bilobar multiple disease. Some advantage in terms of complications and cost-effectiveness have been demonstrated for l-PSH over open PSH in a RCT (14). Few studies to date has addressed whether l-PSH for multiple CRLMs leads to improved outcomes, better survival, or increased likelihood of repeat salvage hepatectomy compared with l-MH (15,16). The results of the published studies are reported and commented here together with personal experience relating to laparoscopic R1vasc surgery. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-12/rc).
Methods
A review of the literature was performed on September 30th, 2021 by searching Medline/PubMed using the terms “Laparoscopic liver resection” and “Parenchymal sparing hepatectomy” from January, 1st 2000 through September, 30th 2021. The titles and abstracts of all pieces of literature were screened for relevance. The research included clinical trials, randomized controlled trials, reviews, systematic reviews and meta-analysis. Books and documents, case reports, letters and commentary articles were excluded. Only publications in English were considered. The search among the references of the articles that were retrieved was also used. Among the articles retrieved, only those focused on l-PSH for CLM and providing results about R1vasc resections were considered eligible for the review. Articles not including laparoscopic R1vasc resections or not providing informations about laparoscopic R1vasc resections were excluded. The detailed search strategy is presented in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | September 30th 2021 |
| Databases and other sources searched | PubMed, Medline |
| The references of the articles that were retrieved | |
| Search terms used | Search terms: “Laparoscopic liver resection” and “Parenchymal sparing hepatectomy” |
| Search strategy of PubMed database: the PubMed Advanced Search Builder was used; filters were applied for article type | |
| Timeframe | Between January, 1st 2000 and September, 30th 2021 |
| Inclusion and exclusion criteria | Inclusion criteria: |
| English-language article | |
| Article types were clinical trials, RCT, reviews, systematic reviews and meta-analysis | |
| Articles focused on l-PSH for CLM | |
| Exclusion criteria: | |
| Study was written in non-English language | |
| Books and documents, case reports, letters and commentary articles | |
| Selection process | The titles and abstracts of all pieces of literature were screened for relevance. Among the retrieved articles, those that did not include laparoscopic R1vasc resections or not providing informations about laparoscopic R1vasc resections were excluded |
RCT, randomized controlled trial; l-PSH, laparoscopic parenchyma sparing hepatectomy; CLM, colorectal liver metastases.
In our personal case series, among a total of 81 laparoscopic liver resections for CLM performed between January 2015 and September 2021, 8 cases met the review criteria mentioned above. Surgical information of these patients are summarized in Table 2.
Table 2
| Patient | Gender | Age | N. lesions | Max size (mm) | Vascular contacts | Surgical procedures | Follow-up (months) | Recurrence |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 81 | 3 | 41 | RHV-MHV | Limited resections S8 + S6 | 8 | Intrahepatic |
| 2 | M | 73 | 1 | 27 | MHV | Limited resection S8 | 19 | No |
| 3 | M | 77 | 1 | 20 | RHV | Limited resection S7 | 17 | Intrahepatic |
| 4 | F | 79 | 1 | 13 | MHV-V8 | Limited resection S4sup-S8 | 5 | No |
| 5 | F | 76 | 1 | 19 | RHV | Limited resection S7 | 11 | Local |
| 6 | M | 43 | 3 | 11 | LHV | Limited resection S2 + S3 | 37 | Intrahepatic |
| 7 | F | 52 | 1 | 11 | LHV | Limited resection S2 | 11 | Intrahepatic |
| 8 | M | 34 | 1 | 29 | LHV-MHV | Limited resection S4sup | 5 | No |
RHV, right hepatic vein; MHV, middle hepatic vein; V8, accessory hepatic vein draining segment 8; LHV, left hepatic vein.
Surgical technique
The preferred approach is with the patient placed in supine position with the legs split and the surgeon standing between the legs. For tumors located in the right postero-lateral segments, a pillow can be placed behind the patient›s back and the bed can be turned sideways.
Intraoperative ultrasound (IOUS) is performed with two main purposes: stage the disease (identification of the target lesions and exclude preoperatively undetected additional lesions) and guide the resection. Laparoscopic and also robotic multifrequency probes (Figure 1) can be used, which today provide high quality images and allow the use of color-flow modalities and ultrasound contrast medium.
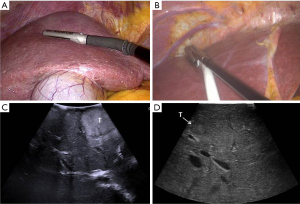
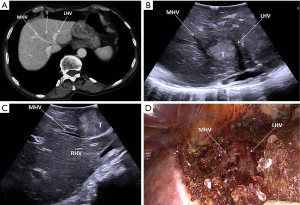
For all resections in the right postero-lateral segments, the right hemiliver must be mobilized sufficiently to allow reaching the target lesion and obtaining a flat resection surface. In case of segment 8 resections, also for deeply located tumors (Figures 2,3), extensive mobilization of the right hemiliver can be avoided. Once the liver mobilization is completed, margins of resections are marked on the liver surface under guidance of IOUS. The IOUS-guided parenchyma-sparing approach has been sistematically described (8), the same criteria apply to laparoscopic surgery: whenever CLM are in contact with major intrahepatic vessels, vascular detachment can be performed if no signs of infiltration are evident at laparoscopic ultrasound (LUS).
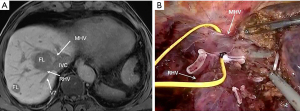
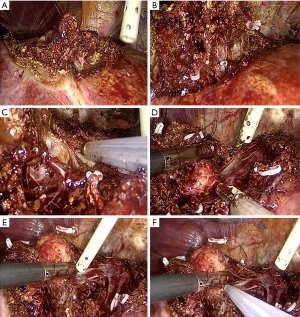
Parenchymal transection can be performed using a sealing device in combination with an ultrasonic surgical aspirator, which is particularly useful, in addition to blunt dissection, also to expose the vessels and detach them from the tumor (Figure 4).
Discussion
Liver resection remains the only treatment for patients with CLM, offering a relatively favorable 5-year overall survival (17), nowadays further improved by the effective association with systemic therapies.
Empowering liver regeneration has been considered the most suitable path to address successfully both surgical radicality and safety in case of extensive neoplastic involvement of the liver, particularly in complex conditions such as multiple bilobar CLM. Following this philosophy the TSH, a temporary debulking surgery splitting into two operations the organ clearance with or without portal vein embolization (PVE), and ALPPS have been introduced (4-6). Liver venous deprivation (LVD) and Radiological Simultaneous Portohepatic Vein Embolization (RASPE) followed by major hepatectomy are the latest techniques aiming to enhance liver regeneration (18,19). All these solutions, boosting the FLR, are conceived for the sacrifice of major vessels, which reduces the chance of redo surgery in case of recurrence compared to the parenchyma sparing approach. Conversely, the philosophy of parenchymal sparing hepatectomies (PSH), avoiding major vessel amputation, increase the surgical salvageability in case of relapses (20,21), resulting in lower rate of surgical mortality without change in disease-specific survival or liver recurrence (22). Indeed, it may be logical that the highest possible integrity of the liver anatomy can offer more technical solutions rather than an “amputated” organ.
Among the predictors of survival after resection, the state of resection margins remains a measure of the oncological adequacy of surgery, still targeted to R0 resections. Furthermore, oncologically suitable tumor-free margin width has progressively reduced from 1-cm to 1-mm (23-26), strengthening the rationale for PSH.
The detachment of colorectal metastases from the vessels, known as R1vasc surgery, also has an anatomical rationale. Major intrahepatic Glissonean pedicles (GP) and hepatic veins (HV) can be considered a kind of barrier separating totally distinct parts of the liver, and they are further separated from the parenchyma not only by the Glissonean sheath and the vascular wall, but also by the Leannec capsule as well as the inferior vena cava itself (27).
In 2008, de Haas et al. reported similar overall survivals for patients undergoing R0 and R1 resections, explaining those findings as the consequence of a more aggressive strategy combining chemotherapy and surgery (28). Subsequently other papers have overcome the paradigm according to which the impossibility of obtaining healthy margins should be a contraindication to surgery (29-32).
More recently, an international survey pointed out that tumor exposure is widely accepted by hepatobiliary surgeons as an alternative approach to non-resection (33) and Adam et al. have shown that R2 surgery can also be proposed in selected cases (34).
As previously mentioned, the parenchyma sparing approach was pushed for multiple bilobar CLM (22,35), commonly resected by an open approach. As for the HCC, the R1vasc policy was adopted according to imaging findings: after an initial encouraging experience (11), further validations on large series with long-term follow-up were subsequently published, showing similar results for R1vasc and R0 resections in term of long-term local control and outcome concerning both the general series (12) and also the subsetting of tumors in contact with major hepatic veins close to the caval confluence (13).
The realization of laparoscopic R1 vascular resection is a demanding procedure, requiring advanced minimally invasive surgical skills, such as the capability to manage large vessels bleeding, in addition to a thorough knowledge of liver anatomy, preoperative imaging and 3D-reconstruction, and a solid experience in intraoperative ultrasound. PSH for deeply located tumors in difficult-to-access areas sometimes requires intricate curved transection planes, which are technically more challenging in the laparoscopic approach in comparison with MH consisting of a single and straight transection plane (36,37).
For all the aforementioned reasons, l-MH (mainly right or left hepatectomy), which extensively sacrifices non-tumorous parenchyma beyond what is required to achieve tumor clearance, is often preferred when a laparoscopic approach is chosen. The technique in fact requires a long laparoscopic learning curve, which could make ineffective most of the advantages of laparoscopy, due to the extension of already long operating times, intraoperative complications and high risk of conversion. Nevertheless, the laparoscopic approach has managed to achieve comparable or improved short-term outcomes in liver surgery compared with the open approach, which has led to its increased implementation in the last 20 years (38-41); however, the role of laparoscopic parenchymal-sparing strategy in the treatment of multiple CRLMs has not been fully defined. L-PSH raises some concerns regarding technical feasibility and oncological outcome, mainly surgical margin status and liver recurrence rates (38). However, large experiences in that field reported similar survival and recurrence rate between patients with resection margins of ≥10 mm and <1 mm (42,43). Moreover, technical advancements and better understanding of hepatic anatomy allow to perform l-PSH even in difficult-to-access lesions (44), resulting in an increasingly frequent reduction in the rate of l-MH.
Some experiences on l-PSH have been published in recent years (14-16) showing favorable results both in terms of short- and long-term outcomes and cost-effectiveness.
The Oslo-CoMet trial (14) is the first published RCT aiming to compare l-PSH and open PSH. No significant differences about R0 and R1 surgical margin status were found between the two groups, and l-PSH showed a significantly lower morbidity rate and were cost-effective, with similar costs but higher QALYs than open liver resection. Nevertheless, no indications about the size, number or location of tumors was specified in the inclusion criteria; only the very limited average number of tumors (1.6 for open-PSH and 1.5 for l-PSH) is reported, suggesting that patient selection for surgery was less aggressive than can generally be done in open surgery. Furthermore, the endpoints did not include long-term oncological outcomes, which remain an open issue.
Two recent single-center retrospective studies compared l-PSH to l-MH (15) and l-PSH for bilobar CLM to laparoscopic liver resection for a single CRLM (16), both showing comparable rates of recurrence-free survival (RFS) and liver-specific RFS. Furthermore, as already reported in various open surgery experiences, l-PSH were associated with lower postoperative morbidity rates compared with l-MH, and l-PSH provided more frequent repeat salvage hepatectomy in case of liver recurrence. In both studies, R0 resections are largely more represented and the R1vasc rate is low, suggesting a large prevalence of patients without vascular contacts undergoing l-PSH. The low median number of resections for single procedure [1, range 1–6 (15) and 3, range 2–4 (16)] confirms the main limit of l-PSH due to the difficulty of the procedures which require prolonged operating times.
In conclusion, allowing for more tolerable large parenchymal removal with similar oncological results and greater chances of repeat liver resections in case of relapse, ultrasound-guided PSH should be the standard of care, even when using the laparoscopic approach, for the treatment of CLM. The limited experiences with the laparoscopic resection of multiple lesions, especially for bilobar disease, remains its main limitation. Based on our experience and published series, l-PSH can be considered indicated in cases where a limited number of resection areas (no more than 3) need to be performed, in order to avoid too long procedures burdened with a greater risk of complications.
Laparoscopic R1 vascular hepatectomy is a technical demanding technique, showing promising results in term of survival, but its evidence in literature remains scarce to draw firm conclusions. However, the technological improvements have played, and will continue to play in the future, a role in developing easier and safer procedures that will increasingly encourage the diffusion of the parenchymal sparing strategy in the modern laparoscopic era. Further studies with larger cohort of patients would be desirable for the future to confirm the oncologic adequacy and reproducibility of this technique.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer review: This article was commissioned by the Guest Editors (Edoardo Rosso and Santiago Azagra) for the series “Focus on Technical Advancement in Mini-invasive HPB Surgery” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-12/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-12/coif). The series “Focus on Technical Advancement in Mini-invasive HPB Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386-422. [Crossref] [PubMed]
- Adam R, De Gramont A, Figueras J, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist 2012;17:1225-39. [Crossref] [PubMed]
- Creasy JM, Sadot E, Koerkamp BG, et al. Actual 10-year survival after hepatic resection of colorectal liver metastases: what factors preclude cure? Surgery 2018;163:1238-44. [Crossref] [PubMed]
- Adam R, Laurent A, Azoulay D, et al. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg 2000;232:777-85. [Crossref] [PubMed]
- Jaeck D, Oussoultzoglou E, Rosso E, et al. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg 2004;240:1037-49; discussion 1049-51. [Crossref] [PubMed]
- Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-14. [Crossref] [PubMed]
- Petrowsky H, Linecker M, Raptis DA, et al. First Long-term Oncologic Results of the ALPPS Procedure in a Large Cohort of Patients With Colorectal Liver Metastases. Ann Surg 2020;272:793-800. [Crossref] [PubMed]
- Torzilli G, Montorsi M, Donadon M, et al. "Radical but conservative" is the main goal for ultrasonography-guided liver resection: prospective validation of this approach. J Am Coll Surg 2005;201:517-28. [Crossref] [PubMed]
- Torzilli G, Montorsi M, Del Fabbro D, et al. Ultrasonographically guided surgical approach to liver tumors involving the hepatic veins close to the caval confluence. Br J Surg 2006;93:1238-46. [Crossref] [PubMed]
- Torzilli G, Del Fabbro D, Palmisano A, et al. Contrast-enhanced intraoperative ultrasonography during hepatectomies for colorectal cancer liver metastases. J Gastrointest Surg 2005;9:1148-53; discussion 1153-4. [Crossref] [PubMed]
- Torzilli G, Donadon M, Palmisano A, et al. Ultrasound guided liver resection: does this approach limit the need for portal vein embolization? Hepatogastroenterology 2009;56:1483-90. [PubMed]
- Viganò L, Procopio F, Cimino MM, et al. Is Tumor Detachment from Vascular Structures Equivalent to R0 Resection in Surgery for Colorectal Liver Metastases? An Observational Cohort. Ann Surg Oncol 2016;23:1352-60. [Crossref] [PubMed]
- Torzilli G, Procopio F, Viganò L, et al. Hepatic vein management in a parenchyma-sparing policy for resecting colorectal liver metastases at the caval confluence. Surgery 2018;163:277-84. [Crossref] [PubMed]
- Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg 2018;267:199-207. [Crossref] [PubMed]
- Okumura S, Tabchouri N, Leung U, et al. Laparoscopic Parenchymal-Sparing Hepatectomy for Multiple Colorectal Liver Metastases Improves Outcomes and Salvageability: A Propensity Score-Matched Analysis. Ann Surg Oncol 2019;26:4576-86. [Crossref] [PubMed]
- D'Hondt M, Pironet Z, Parmentier I, et al. One-stage laparoscopic parenchymal sparing liver resection for bilobar colorectal liver metastases: safety, recurrence patterns and oncologic outcomes. Surg Endosc 2022;36:1018-26. [Crossref] [PubMed]
- Minagawa M, Makuuchi M, Torzilli G, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg 2000;231:487-99. [Crossref] [PubMed]
- Guiu B, Quenet F, Escal L, et al. Extended liver venous deprivation before major hepatectomy induces marked and very rapid increase in future liver remnant function. Eur Radiol 2017;27:3343-52. [Crossref] [PubMed]
- Laurent C, Fernandez B, Marichez A, et al. Radiological Simultaneous Portohepatic Vein Embolization (RASPE) Before Major Hepatectomy: A Better Way to Optimize Liver Hypertrophy Compared to Portal Vein Embolization. Ann Surg 2020;272:199-205. [Crossref] [PubMed]
- Mise Y, Aloia TA, Brudvik KW, et al. Parenchymal-sparing Hepatectomy in Colorectal Liver Metastasis Improves Salvageability and Survival. Ann Surg 2016;263:146-52. [Crossref] [PubMed]
- Hosokawa I, Allard MA, Mirza DF, et al. Outcomes of parenchyma-preserving hepatectomy and right hepatectomy for solitary small colorectal liver metastasis: A LiverMetSurvey study. Surgery 2017;162:223-32. [Crossref] [PubMed]
- Gold JS, Are C, Kornprat P, et al. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in the oncologic outcome: trends in treatment over time in 440 patients. Ann Surg 2008;247:109-17. [Crossref] [PubMed]
- Kokudo N, Miki Y, Sugai S, et al. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: minimum surgical margins for successful resection. Arch Surg 2002;137:833-40. [Crossref] [PubMed]
- Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005;241:715-22, discussion 722-4. [Crossref] [PubMed]
- Cady B, McDermott WV. Major hepatic resection for metachronous metastases from colon cancer. Ann Surg 1985;201:204-9. [Crossref] [PubMed]
- Elias D, Cavalcanti A, Sabourin JC, et al. Resection of liver metastases from colorectal cancer: the real impact of the surgical margin. Eur J Surg Oncol 1998;24:174-9. [Crossref] [PubMed]
- Sugioka A, Kato Y, Tanahashi Y. Systematic extrahepatic Glissonean pedicle isolation for anatomical liver resection based on Laennec's capsule: proposal of a novel comprehensive surgical anatomy of the liver. J Hepatobiliary Pancreat Sci 2017;24:17-23. [Crossref] [PubMed]
- de Haas RJ, Wicherts DA, Flores E, et al. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg 2008;248:626-37. [Crossref] [PubMed]
- Eveno C, Karoui M, Gayat E, et al. Liver resection for colorectal liver metastases with peri-operative chemotherapy: oncological results of R1 resections. HPB (Oxford) 2013;15:359-64. [Crossref] [PubMed]
- Ayez N, Lalmahomed ZS, Eggermont AM, et al. Outcome of microscopic incomplete resection (R1) of colorectal liver metastases in the era of neoadjuvant chemotherapy. Ann Surg Oncol 2012;19:1618-27. [Crossref] [PubMed]
- Andreou A, Aloia TA, Brouquet A, et al. Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg 2013;257:1079-88. [Crossref] [PubMed]
- Tranchart H, Chirica M, Faron M, et al. Prognostic impact of positive surgical margins after resection of colorectal cancer liver metastases: reappraisal in the era of modern chemotherapy. World J Surg 2013;37:2647-54. [Crossref] [PubMed]
- Viganò L, Costa G, Cimino MM, et al. R1 Resection for Colorectal Liver Metastases: a Survey Questioning Surgeons about Its Incidence, Clinical Impact, and Management. J Gastrointest Surg 2018;22:1752-63. [Crossref] [PubMed]
- Adam R, Kitano Y, Abdelrafee A, et al. Debulking surgery for colorectal liver metastases: Foolish or chance? Surg Oncol 2020;33:266-9. [Crossref] [PubMed]
- Torzilli G, Procopio F, Botea F, et al. One-stage ultrasonographically guided hepatectomy for multiple bilobar colorectal metastases: a feasible and effective alternative to the 2-stage approach. Surgery 2009;146:60-71. [Crossref] [PubMed]
- Kawaguchi Y, Fuks D, Kokudo N, et al. Difficulty of Laparoscopic Liver Resection: Proposal for a New Classification. Ann Surg 2018;267:13-7. [Crossref] [PubMed]
- Hasegawa Y, Wakabayashi G, Nitta H, et al. A novel model for prediction of pure laparoscopic liver resection surgical difficulty. Surg Endosc 2017;31:5356-63. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831-41. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Ciria R, Cherqui D, Geller DA, et al. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg 2016;263:761-77. [Crossref] [PubMed]
- Postriganova N, Kazaryan AM, Røsok BI, et al. Margin status after laparoscopic resection of colorectal liver metastases: does a narrow resection margin have an influence on survival and local recurrence? HPB (Oxford) 2014;16:822-9. [Crossref] [PubMed]
- Kazaryan AM, Aghayan DL, Barkhatov LI, et al. Laparoscopic Multiple Parenchyma-sparing Concomitant Liver Resections for Colorectal Liver Metastases. Surg Laparosc Endosc Percutan Tech 2019;29:187-93. [Crossref] [PubMed]
- Ishizawa T, Gumbs AA, Kokudo N, et al. Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg 2012;256:959-64. [Crossref] [PubMed]
Cite this article as: Del Fabbro D, Giuffrida M, Dalla Valle R, Torzilli G. Ultrasound-guided parenchima-sparing liver surgery: is it feasible by laparoscopy? Personal experience and narrative review of the literature. Dig Med Res 2022;5:32.


