
New technique to increase resection margins during mini-invasive liver resection: a propensity score-matched study
Introduction
Hepatectomy remains the gold standard treatment for liver malignancies, offering the best chance for long term survival (1).
However, despite liver resection, several factors may affect long-term survival. Some of them are related to the tumor inner biology, such as tumoral size and numbers, increased tumoral markers serum level and mutated KRAS; some others are related to the surgical procedure itself, such as surgical margins (R0 vs. R1 resection) (2). It is well known that the R1 resection negatively impact disease-free and overall survival (OS) rates in all liver malignancies, therefore, whenever possible, the gold standard of treatment is to achieve enough margins to decrease the potential recurrence (3).
However, depending on tumor malignancy, length margin may vary. Furthermore, currently, definition of margin status still lacks of uniformity. For instance, because of the tendence of hepatocarcinoma for vascular invasion as well as metastatic spread along the portal venous system, a margin wider than 1 cm is generically desirable (4). For hepatocarcinoma, several studies found that the 1 cm margin can be an independent predictor of disease-free survival for tumors >2 cm (4). In this case, even anatomic resection has been proposed as a means to provide increased resection margins and survival (5).
Many studies have addressed surgical margins for colorectal liver metastases (CRLM), even if it still remains an issue among scholars (3). Some series concluded that a minimum margin higher than 1 mm is associated with a better prognosis and that a margin >1 cm achieved an even better prognosis (3). For CRLM, resected tumors with margin within 1 cm are considered to be at potentially risk of recurrence (3).
Furthermore, some authors for tumors involving hepatic veins, suggested that an R1 resection with detachment of CRLM from vessels, defined as vascular R1 resection, achieve an acceptable survival compared with local treatment without resection (6,7).
However, the extent of the liver resection should consider what may be the underlying hepatic dysfunction and functional hepatic reserve of the patient. Sometime a proper R1 resection, that may require extended resection or vascular resection, cannot be achieved with acceptable morbidity. In addition, in the current era of neoadjuvant chemotherapy, most of the cases underwent preoperative treatment, therefore, the R1 resection margins concept should be reconsidered in light of the recently highly effective regimens.
In cases with important underlying hepatic dysfunction or with large tumors, to achieve wide margins may be challenging, or aiming to increase resection margins in all liver resection, several techniques have been described (8-11).
The application of the radiofrequency (RF) to the liver during resection is a relatively new technique that use similar currents (around 400 kHz) compared with the traditional RF ablation commonly used for the local treatment of liver nodules, but, with different aim and approach. The RF ablation is based on delivering energy in the tumor itself aiming to ablate it without its resection, therefore, with potential worst survival rate. Conversely, RF applied along liver transection has the aim to resect the tumor in a bloodless fashion applying margin ablation to the remnant liver, as well.
In previous reports, we have been able to show the positive impact of recurrence rate for primary and secondary liver malignancies by using a specific RF device for liver transection (12).
The aim of this study is to investigate the impact of RF assisted transection on local recurrence (LR) rate for high risk cases (margins <1 cm) focusing selectively on CRLM resected laparoscopically at our center. We present the following article in accordance with the STROBE reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-106/rc).
Methods
Patient eligibility and data collection
A retrospective analysis from clinical data of patients underwent liver resection for CRLM from September 2006 to September 2020 at our center was done. The study protocol was approved by the Clinical Research and Ethics Committee of Hospital del Mar, Barcelona, Spain (ID 2020-9397) (13). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Clinical Research and Ethics Committee of Hospital del Mar (Barcelona, Spain) (No. 2020-9397) and individual consent for this retrospective analysis was waived.
Inclusion criteria are: (I) patients older than 18 years old; (II) CRLM removed by laparoscopic liver resection. Exclusion criteria are: (I) benign lesions; (II) non-colorectal metastases; (III) absence of extrahepatic metastases; (IV) patients treated with only RF ablation; and (V) incomplete clinical data. We included only patients with a margin width 1 cm. Patients were divided in two groups depending on the fashion as haemostasis was performed: with conventional haemostatic devices (Control group), or with an additional coagulation area by means of an RF-based device (RF group). In two groups hepatic parenchymal transection was performed by the Cavitron ultrasonic surgical aspirator, stapler transection or Ligasure. The final haemostasis was achieved in the Control group through stitches, clips, monopolar or bipolar energy device and Ligasure. In the RF group, haemostasis was performed with the Coolingbis device (14-16). After liver resection in the RF group, the RF energy were applied and repeated if necessary until no further spurting hemorrhage and to increase the safety margin width (up to 1 cm). Coolingbis was used according to the habits of the surgeons in terms of hemostatic effectiveness only. All surgeries are performed by the same team of surgeon.
Outcome indicators
The follow-up is done starting from almost 30 days from surgery and then every 6 months after hospitalization with blood tests and imaging (CT scan and MRI). Data are obtained from our prospective database recorded up to September, 2020.
Main end-point is LR and secondary end-points included clinical variables, OS, and postoperative complications. LR is defined as the presence of any growing or enhancing tumour along the surgical margin or the presence of tumor cells at the surgical margin detected by microscopical final examination. Specimen-tumor margin is defined as the shortest distance from the edge of the tumor to the line of transection measured in millimetres at final pathological study. Survival are defined from the operation up to the latest follow-up. Post-operative complications are classified according to Clavien-Dindo and comprehensive complcation index (CCI) (17,18); a severe complication is defined when ≥3 was used for comparing cumulative severity complications between groups (17). Complications were recorded up to 90 days from surgery. The standard “50-50 criteria” defined the post-operative hepatic failure (19).
Statistical analysis
To balance the groups preoperative data, we used propensity score matching (PSM) to perform 1:1 matching between the two groups. The PSM system was based on logistic regression with various matching variables, including age, sex, number of tumours, size of the biggest tumour with a calliper value of 0.03. The Kolmogorov-Smirnov test is used to check the normality of the data and the Levene test for equality of variances. Categorical variables are reported as number and percentage, and continuous variables are reported as mean and standard deviation (SD) when the distribution was considered normal or using the median and interquartile range (IQR). Comparison were made through the Mann-Whitney U test or Student’s t-test before PSM. The Wilcoxon test or Student’s t-test is performed after PSM. Chi-square test and McNemar’s test were used to compare categorical variables before and after PSM respectively.
Survival data are calculated through Kaplan-Meier, and the log-rank test is used to compare survival. Hazards ratio (HRs) with 95% confidence intervals (CI) was used to measure the association between additional margin coagulation and LR. P value lower than 0.05 is considered statistically significant. Analyses were performed with SPSS version 25.0.
Results
Patients characteristics
A total of 283 patients with liver tumors who underwent the ptectomy between September 2006 and September 2020 were evaluated (Figure 1). According to the inclusion and exclusion criteria, finally, 123 patients were selected (160 cases are excluded for reasons including distance from the tumor to resection margin ≥10 mm, n=63; primary liver tumor, n=97). There were 52 (42.3%) patients in the Control group and 71 (57.7%) patients in the RF group. Patient baseline and preoperative characteristics of the two groups before and after PSM are summarized in Table 1. All patients completed follow-up The baseline patients’ characteristics before PSM showed relevant differences in surgical procedure (P=0.019) (Table 1). After 1:1 PSM, a total of 51 cases in both groups with similar baseline characteristics have been included (Table 1).
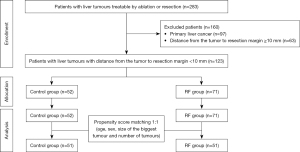
Table 1
| Baseline characteristics | Before propensity score-matching | After propensity score-matching | |||||
|---|---|---|---|---|---|---|---|
| Control group (n=52) | RF group (n=71) | P value* | Control group (n=51) | RF group (n=51) | P value** | ||
| Male sex | 33 (63.5%) | 43 (60.6%) | 0.744a | 33 (32.4%) | 30 (29.4%) | 0.700d | |
| Age (years), mean (SD) | 68.4 (10.3) | 66.9 (11.6) | 0.437b | 68.4 (10.4) | 66.3 (11.1) | 0.318e | |
| Number of metastases | 0.970a | 0.261d | |||||
| Solitary tumors | 30 (57.7%) | 41 (57.7%) | 29 (28.4%) | 28 (27.5%) | |||
| 2 to 3 tumors | 15 (28.8%) | 19 (26.8%) | 15 (14.7%) | 16 (15.7%) | |||
| 4 to 5 tumors | 5 (9.6%) | 7 (9.9%) | 5 (4.9%) | 4 (3.9%) | |||
| ≥6 tumors | 2 (3.8%) | 4 (5.6%) | 2 (2.0%) | 3 (2.9%) | |||
| Size of the biggest tumor (cm), median (IQR) | 2.9 (22.0–0.4) | 2.5 (8.5–0.4) | 0.236c | 2.9 (0.4–10.0) | 2.2 (0.4–8.0) | 0.320f | |
| Distance to resection margin | 0.883a | 0.380d | |||||
| 0 mm | 20 (38.5%) | 27 (38.0%) | 20 (19.6%) | 20 (19.6%) | |||
| 1–4 mm | 20 (38.5%) | 25 (35.2%) | 19 (18.6%) | 17 (16.7%) | |||
| 5–9 mm | 12 (22.1%) | 19 (26.8%) | 12 (11.8%) | 14 (13.7%) | |||
| Surgical data | |||||||
| Operative procedure | 0.019a | 1.000d | |||||
| Right hepatectomy | 11 (21.2%) | 6 (8.5%) | 10 (9.8%) | 3 (2.9%) | |||
| Left hepatectomy | 2 (3.8%) | 7 (9.9%) | 2 (2.0%) | 5 (4.9%) | |||
| Segmentectomy/bisegmentectomy | 11 (21.2%) | 6 (8.5%) | 11 (10.8%) | 4 (3.9%) | |||
| Atypical resection | 28 (53.8%) | 49 (69.0%) | 28 (27.5%) | 36 (35.3%) | |||
| Other liver resection | 0 (0.0%) | 3 (4.2%) | 0 (0.0%) | 3 (2.9%) | |||
| Laparoscopic approach | 22 (42.3%) | 38 (53.5%) | 0.219a | 22 (21.6%) | 26 (25.5%) | 0.523d | |
| Pringle maneuver (min), median (IQR) | 0 (0.0–60.0) | 2 (0.0–86.0) | 0.203c | 0 (0.0–60.0) | 1 (0.0–83.0) | 0.914f | |
Differences in variables were considered to be significant at a threshold of P<0.05. Cumulative length of Pringle maneuver (min). *, P value for the difference between Control group and RF group before propensity score-matching; a, chi-squared test; b, Student’s t-test; c, Mann-Whitney U test; **, P value for the difference between Control group and RF group after propensity score-matching; d, McNemar test; e, paired samples Student’s t-test; f, Wilcoxon test. RF, radiofrequency; SD, standard deviation; IQR, interquartile range.
LR analysis
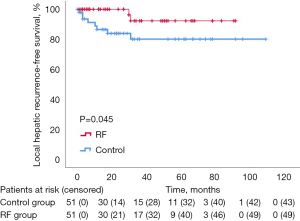
Regarding the primary endpoint of the study, overall 10 (9.8%) out of 102 patients developed LR, that was significantly higher in the Control group then the RF group [8 (7.84%) vs. 2 (1.9%) patients, P=0.046]. The 1-, 3-, and 5-year LR rate were 86.5%, 83.9% and 80.1%, respectively, in the Control group and 100%, 100% and 92.3%, respectively, in the RF group (P=0.045) (Figure 2). The RF group was associated with reduced LR (HR =4.69; 95% CI: 0.983–22.40; P=0.053).
Postoperative outcomes and OS analysis
Th overall stay resulted lower in the RF group compared with the Control group (median, 8 vs. 5 days, P=0.009) (Table 2). The rates of general complications in terms of liver failure, bike leak or abdominal abscesses did not differ between the groups. Mortality was similar among the groups.
Table 2
| Complications | Control group (n=51) | RF group (n=51) | Total | P value |
|---|---|---|---|---|
| Morbility | 27 (52.9%) | 17 (33.3%) | 44 (43.1%) | 0.078a |
| Abscess | 11 (21.6%) | 4 (7.8%) | 15 (14.7%) | 0.118a |
| Biliary leak | 1 (2.0%) | 5 (5.9%) | 6 (5.9%) | 0.625a |
| Hemoperitoneum | 0 (0.0%) | 1 (2.0%) | 1 (1.0%) | 1.000a |
| Liver failure | 3 (6.0%) | 4 (8.0%) | 7 (6.9%) | 1.000a |
| Wound infection | 4 (7.8%) | 3 (5.9%) | 7 (6.9%) | 1.000a |
| Pneumonia | 0 (0.0%) | 1 (1.0%) | 1 (1.0%) | 1.000a |
| Other complications | 17 (33.3%) | 12 (23.5%) | 29 (28.4%) | 0.424a |
| Blood transfusion | 6 (6.0%) | 4 (4.0%) | 10 (9.8%) | 0.754a |
| Red packed cells transfusion, median (IQR) | 0 (0–7) | 0 (0–4) | – | 0.566b |
| Clavien-Dindo grades* | 0.164a | |||
| No | 23 (45.1%) | 33 (64.7%) | 17 (54.9%) | |
| 1 | 5 (9.8%) | 4 (7.8%) | 9 (8.8%) | |
| 2 | 7 (25.5%) | 4 (7.8%) | 11 (10.8%) | |
| 3a | 13 (39.0%) | 10 (32.3%) | 23 (22.5%) | |
| 3b | 1 (2.0%) | 3 (5.9%) | 4 (3.9%) | |
| 4a | 1 (2.0%) | 1 (2.0%) | 2 (2.0%) | |
| 4b | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| 5 | 1 (2.0%) | 1 (2.0%) | 2 (2.0%) | |
| CCI score, median (IQR) | 8.7 (0–100) | 0 (0–100) | – | 0.164b |
| Reoperation* | 2 (3.9%) | 2 (3.9%) | 4 (7.8%) | 1.000a |
| Length of stay (days), median (IQR) | 8 (1–31) | 5 (1–49) | – | 0.009b |
| 90-day mortality | 1 (2.0%) | 1 (2.0%) | 2 (2.0%) | 1.000a |
Data as absolute numbers and percentages in parenthesis unless otherwise stated. Statistical differences were considered to be significant at a threshold of P<0.05. a, McNemar test; b, Wilcoxon test; *, within 90 days. RF, radiofrequency; IQR, interquartile range; CCI, comprehensive complcation index.
Twenty-three of 51 (45.1%) patients in the Control group and 10 of 51 (19.6%) in the RF group had died after a median follow-up period of 60 months. The 1-, 3-, and 5-year global cumulative OS were 93.5%, 71.5% and 37.5%, respectively, in the Control group and 95.4%, 87.4% and 67.8%, respectively, in the RF group (P=0.012).
Discussion
As involved margins are related with higher chance of recurrence, the achievement of enough resection margins for liver malignancies is one of the most important goals during liver transection (20).
After curative resection, liver represents the most frequent site for malignant recurrence for CRLM, occurring up to 50% within the first 2 years from resection (1).
As it has been defined above, margin still represent an important prognostic factor. Some decades ago, at the beginning of laparoscopic liver resection development, it has been claimed that minimally invasive approach may increase affected margin rate (1). However, with increased experience, it has been lately showed that traditional open and minimally approach entails similar pathological outcomes, but with better recovery (21).
Nevertheless, the type of approach, intraoperatively, the assessment of the required surgical margin is challenging and for this reason the rate of R1 resection rate after liver resection for CRLM can achieve a rate up to almost 30% even in experienced centers (1,2). Therefore, it is paramount for a surgeon achieve enough margins to assure a low recurrence rate after resection.
Giving this background, we analyzed in a matched-pair analysis the impact of the RF assisted liver transection on surgical margin compared with the standard technique. The findings from this study indicated that LR for the RF group is significantly lower compared with the standard technique being lower compared with the standard Control group (7.84% vs. 1.9%). This data is further confirmed by the higher OS of the RF group after liver resection, as it is shown in Figure 1. This result can be explained by the additional ablation effect of RF device used in this study.
In this study we decided to include only CRLM patients at higher risk to develop recurrence after resection, being <1 cm the selection criteria. This decision belongs from the results of a recent meta-analysis for CRLM which concluded that, whenever possible, according to previous studies, surgeons should obtain a minimum 1-cm margin along liver resection (3). Whenever a new technology is introduced, it is paramount to assess its safety and effectiveness, as well. As depicted in the Table 2, our results showed that the RF assisted liver transection is a safe and affective technique. Main immediate post-operative outcomes are similar in both groups.
We reckon limitations of the present study. It is a retrospective and single-center study with a relatively small sample size; therefore, the selection bias could not be entirely eliminated. However, biases after PSM assessment were minimized.
In conclusion, our results provide rationale to study the effect of RF liver transection device among CRLM patients in future prospective randomized studies.
Acknowledgments
Funding: This work was partially supported by the Spanish Ministerio de Ciencia, Innovación y Universidades under “Programa Estatal de I+D+i Orientada a los Retos de la Sociedad” (grants No. RTI2018-094357-B-C21 and No. RTI2018-094357-B-C22).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Edoardo Rosso and Juan Santiago Azagra) for the series “Focus on Technical Advancement in Mini-invasive HPB Surgery” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-106/rc
Data Sharing Statement: Available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-106/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-106/coif). The series “Focus on Technical Advancement in Mini-invasive HPB Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest or financial ties to disclose.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Clinical Research and Ethics Committee of Hospital del Mar (Barcelona, Spain) (No. 2020-9397) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chandra R, Karalis JD, Liu C, et al. The Colorectal Cancer Tumor Microenvironment and Its Impact on Liver and Lung Metastasis. Cancers (Basel) 2021;13:6206. [Crossref] [PubMed]
- Vidal-Vanaclocha F, Crende O, García de Durango C, et al. Liver prometastatic reaction: Stimulating factors and responsive cancer phenotypes. Semin Cancer Biol 2021;71:122-33. [Crossref] [PubMed]
- Liu W, Sun Y, Zhang L, et al. Negative surgical margin improved long-term survival of colorectal cancer liver metastases after hepatic resection: a systematic review and meta-analysis. Int J Colorectal Dis 2015;30:1365-73. [Crossref] [PubMed]
- Famularo S, Piardi T, Molfino S, et al. Factors Affecting Local and Intra Hepatic Distant Recurrence After Surgery for Hcc: An Alternative Perspective on Microvascular Invasion and Satellitosis - A Western European Multicentre Study. J Gastrointest Surg 2021;25:104-11. [Crossref] [PubMed]
- Liu H, Hu FJ, Li H, et al. Anatomical vs nonanatomical liver resection for solitary hepatocellular carcinoma: A systematic review and meta-analysis. World J Gastrointest Oncol 2021;13:1833-46. [Crossref] [PubMed]
- Viganò L, Costa G, Cimino MM, et al. R1 Resection for Colorectal Liver Metastases: a Survey Questioning Surgeons about Its Incidence, Clinical Impact, and Management. J Gastrointest Surg 2018;22:1752-63. [Crossref] [PubMed]
- Torzilli G, Adam R, Viganò L, et al. Surgery of Colorectal Liver Metastases: Pushing the Limits. Liver Cancer 2016;6:80-9. [Crossref] [PubMed]
- Weber JC, Navarra G, Jiao LR, et al. New technique for liver resection using heat coagulative necrosis. Ann Surg 2002;236:560-3. [Crossref] [PubMed]
- Hering J, Garrean S, Saied A, et al. Use of radiofrequency hepatic parenchymal transection device in hepatic hemangioma resection: early experience and lessons learned. HPB (Oxford) 2007;9:319-23. [Crossref] [PubMed]
- Haghighi KS, Wang F, King J, et al. In-line radiofrequency ablation to minimize blood loss in hepatic parenchymal transection. Am J Surg 2005;190:43-7. [Crossref] [PubMed]
- Clancy TE, Swanson RS. Laparoscopic radiofrequency-assisted liver resection (LRR): a report of two cases. Dig Dis Sci 2005;50:2259-62. [Crossref] [PubMed]
- Villamonte M, Burdío F, Pueyo E, et al. The impact of additional margin coagulation with radiofrequency in liver resections with subcentimetric margin: can we improve the oncological results? A propensity score matching study. Eur J Surg Oncol 2022;48:82-8. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495-9. [Crossref] [PubMed]
- Burdío F, Grande L, Berjano E, et al. A new single-instrument technique for parenchyma division and hemostasis in liver resection: a clinical feasibility study. Am J Surg 2010;200:e75-80. [Crossref] [PubMed]
- Burdío F, Navarro A, Berjano E, et al. A radiofrequency-assisted device for bloodless rapid transection of the liver: a comparative study in a pig liver model. Eur J Surg Oncol 2008;34:599-605. [Crossref] [PubMed]
- Burdío F, Berjano EJ, Navarro A, et al. Research and development of a new RF-assisted device for bloodless rapid transection of the liver: computational modeling and in vivo experiments. Biomed Eng Online 2009;8:6. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1-7. [Crossref] [PubMed]
- Balzan S, Belghiti J, Farges O, et al. The "50-50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 2005;242:824-8. [Crossref] [PubMed]
- Chin KM, Prieto M, Cheong CK, et al. Outcomes after curative therapy for hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: a meta-analysis and review of current literature. HPB (Oxford) 2021;23:1164-74. [Crossref] [PubMed]
- Ielpo B, Pittau G, Ciacio O, et al. Standardized laparoscopic right hepatic lobe mobilization. J Hepatobiliary Pancreat Sci 2022;29:e30-2. [Crossref] [PubMed]
Cite this article as: Villamonte M, Burdío F, Sánchez-Velázquez P, Pérez M, Martinez A, Ielpo B. New technique to increase resection margins during mini-invasive liver resection: a propensity score-matched study. Dig Med Res 2022;5:23.


