
Management of an aberrant right hepatic artery arising from the superior mesenteric artery during minimally-invasive pancreatoduodenectomy—a narrative review
Introduction
Since its first description in 1994 by Gagner et al. (1), minimally-invasive pancreatoduodenectomy (MIPD) has represented one of the most challenging procedures in the setting of laparoscopic and then in robotic surgery.
As a matter of fact, minimally-invasive (MI) approach increases the technical complexity to an intervention which is already burdened by moderate postoperative mortality and elevated postoperative morbidity (2-4). In spite of this, in the last three decades MIPD has gained increasing diffusion in tertiary centers and a growing number of studies on this topic have been published so far. As the interest toward MIPD grows, more attention is given to the management of some technical aspects that increase the intricacy of the procedure and interfere with the possibility to perform it laparoscopically.
The advantages of MIPD in postoperative and oncological outcomes are better than those obtained with the open approach (5-9) in term of shorter postoperative length of stay and accuracy in lymphadenectomy with similar mortality and overall survival, but a precise knowledge of the vascular anatomy is mandatory to avoid inadvertent vascular injury (10).
The vascular supply to liver is known to be often characterized by many possible anatomical variations (11) whose management must be mastered with ease by the pancreatic surgeon in either open or MI approach not only in order to obtain a R0 resection in case of a contact between the aberrant artery and the tumor but also to avoid any inadvertent injury to any aberrant hepatic artery.
An aberrant hepatic artery can be accessory or replaced (12). An accessory hepatic artery (aHA) is a vessel that arises from an uncommon origin and supplies a portion of the liver along with another hepatic artery. The embolization or surgical ligation of an aHA may have no consequence on the vascular supply to the liver.
A replaced hepatic artery (rHA) is a vessel that arises from an anomalous origin and supplies a portion of the liver, that is not supplied otherwise by any other artery. A careful evaluation before surgery is mandatory to identify this vascular variant in order to avoid any postoperative complications.
The aim of this manuscript is to resume the surgical strategy when performing a MIPD, based on a review of the current available literature and our experience, face to an aberrant right hepatic artery (RHA) arising from the superior mesenteric artery (SMA). We present the following article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-8/rc).
Methods
We conducted a review of the current literature till the 15th January 2022; the searched databases were Scopus, Embase, Pubmed and Cochrane library. Two authors (ACa, ACh) conducted the research independently. An additional hand-on search in the references lists of the reviews identified on this subject completed the articles research (ME). Any disagreement in review process was solved by a third party (ER, AI), who supervised the review process.
All case series, cohort studies and case reports describing the preoperative strategy, intraoperative procedure, and outcomes after a robotic or laparoscopic PD in presence of an aberrant RHA originating from the SMA were included; moreover, articles describing peculiar preoperative strategies to menage the aberrant RHA before open PD were also included. Studies in non-English language or for whom the full-text was not available were excluded. The characteristics of the review process are summarized in Table 1.
Table 1
| Item | Specification |
|---|---|
| Date of search | Jan. 16, 2022 |
| Database and other sources searched | Scopus, Embase, PubMed, Cochrane Library |
| Search terms used | PubMed: “((pancreatoduodenectomy) OR (duodenopancreatectomy)) AND ((laparoscopic) OR (robotic) OR (minimally-invasive)) AND (right) AND ((accessor*) OR (replac*)” |
| Timeframe | No time restrictions up to Jan. 15, 2022 |
| Inclusion and exclusion criteria | Inclusion criteria: cohort studies, case series and case reports describing pre- and intraoperative strategy and outcomes after MIPD in presence of an aberrant RHA arising from the SMA |
| Exclusion criteria: non-English literature; no full-text available | |
| Selection process | ACa and ACh performed the database search, ME performed an additiona hand-on search, ER and AI supervised the review process and solved review disagreements |
Main body
Preoperative strategy
Aberrant RHAs are quite common and their identification before surgery is of paramount importance to set up the best surgical strategy to avoid any injury to arterial supply to the liver with consequent ischemia-related complications during MIPD. The most dreadful consequences of an acute partial ischemia of liver parenchyma consists in liver abscess and biliary ischemic complications; in addition, injury to hepatic arteries during surgery may be responsible for thrombosis or pseudoaneurysm formation that can lead to postoperative hemorrhage.
A recent study by Yan et al. (12) reporting anatomical definition of liver arterial supply based on contrast-enhanced CT-scan, identified the presence of either an accessory RHA (aRHA) or a replaced RHA (rRHA) in 33.6% of individuals. An aberrant RHA can have many different characteristics depending on its origin, its path toward the liver, and its eventual contact or invasion by the tumor. While an aRHA is defined as an additional RHA coexisting with a modal RHA, a rRHA originates from an unusual artery completely replacing the modal RHA.
The preoperative precise definition of vascular anatomy for each single case represents the first step for a safe MIPD (13) and it is mainly assessed through contrast-enhanced CT-scan. Even though triphasic CT-scan is easily accessible and has few contraindications, its most evident drawback is represented by the fact that it does not provides dynamic imaging of the arterial vascular supply.
Although no study reports its application before MI and open PD with this target, angiography could be used to complement information obtained with CT-scan imaging. Through direct contrast injection followed by active image records, angiography permits to confirm the presence of vascular aberrancies; moreover, the dynamic visualization of the arterial flow gives additional information concerning which is the preferential path for the right hepatic lobe vascular supply. Furthermore, selective catheterization with temporary vascular occlusion or injection of the contrast agent under variable degrees of pressure can simulate what it would happen in case of aberrant RHA ligation with the eventual identification of arterial shunts through the hilar plate. Some authors have reported the use of preoperative angiography for aberrant RHA embolization before open PD. The cases reported by Ishikawa et al. (14), Cloyd et al. (15), and El Amrani et al. (16) describe the successful embolization of a rRHA originating from the SMA in a total of 5 patients. The aim of this procedure was to stimulate arterial shunts formation in order to perform PD with aberrant RHA ligation without compromising liver arterial supply. Shunts’ opening was confirmed by preoperative CT-scan 3 weeks after angioembolization with no evidence of liver ischemia in any of the included patients. Only in one study, right liver lobe revascularization through arterial shunts was confirmed only with an immediate post embolization angiography. No embolization and postoperative related ischemia complications were reported. The largest case series on this subject, however, is a bi-centric cohort retrospective study by Marichez et al. (17) that describes the results of 16 preoperative angioembolization of a rRHA before laparotomic PD with rRHA ligation. All patients received a triphasic CT-scan one day after angioembolization confirming adequate right liver vascularization.
Intraoperative strategy
Once vascular anatomy is correctly defined, the initial intraoperative attitude should be centered on the identification and isolation of the aberrant RHA.
Most of the descriptions of MIPD in presence of this anatomical peculiarity delineate a posterior approach with rapid identification of the SMA as the most suitable strategy in this situation (18-23). The intervention starts with a full Kocher maneuver and the exposition of the inferior vena cava. Then, the left renal vein is isolated and the SMA is isolated at its origin from the aorta. This, in turn, allows to recognize any possible aberrant artery arising from the SMA avoiding its inadvertent ligation. Once the origin of the aberrant RHA is isolated, the next step is to identify it distally in the hepatic pedicle. The access to the aberrant RHA in the liver hilum is gained isolating and dividing the common bile duct, usually after the cholecystectomy. The artery is then encircled in the liver pedicle and then fully mobilized avoiding any manipulation and traction that can lead to subsequent thrombosis. PD can be then completed with the division of the stomach and the first jejunal loop, pancreatic body division above the superior mesenteric vein and pancreatoduodenal bloc resection. The preemptive identification of the variant RHA helps reducing possible unintentional lesions and leaves the possibility to take the best choice in terms of surgical strategy with regard to the artery.
Nguyen et al. (24) were the first to publish their experience of MIPD in the presence of an aberrant RHA. In a cohort of 30 patients with vascular anomalies undergoing robotic PD, 15 were found to have a rRHA from the SMA. A conservative approach toward the aberrant RHA was applied in all cases and no differences in postoperative and oncological outcomes were identified comparing patients with and without vascular anomalies. The same results were reported by Wang et al. (25) in 58 patients with an aRHA or rRHA undergoing a laparoscopic PD with preservation of the aberrant artery. No differences in term of conversion rate, intraoperative outcomes such as operative time and estimated blood loss, post-PD complications, number of harvested lymph nodes, and R0 resection margins was also highlighted by Kim et al. (26) when comparing patients with aberrant RHA to patients with normal vascular anatomy undergoing robotic PD. In this cohort, 11 patients underwent successful rRHA conservative management while 2 out of 4 with an aRHA needed aberrant RHA ligation and division; one of them experimented an initial postoperative increase in liver enzymes that rapidly normalized with no other complications.
Considering oncological outcomes in the setting of MIPD in patients with vascular anomalies, Giani et al. (27) identified a significant increased median number of harvested lymph nodes in the aberrant vascular group compared to the modal vascular anatomy group. They analyzed 9 patients with a rRHA and 1 with an aRHA from the SMA undergoing laparoscopic PD but did not report how the aberrant arteries had been managed. Lastly, Zhang et al. (28) in their comparative study including 22 patients with aberrant RHA (12 aRHA and 10 rRHA), found a significant increased operative time for this patient compared to those with a modal anatomy. The increased operative time was related to the time spent to spare the RHA arising from the SMA.
Focusing on the necessity to resect or conserve the aberrant RHA, although the most frequently described behavior is conservative, the anatomy of the tumor deeply affects the choice. In a cohort of 25 patients with a rRHA undergoing laparotomic PD, Okada et al. (29) report a significantly different rate of R0/R1 margins depending on the distance between the tumor and the aberrant RHA with an increased incidence of R1 margins for tumors with ≤10 mm interspace between the tumor and the rRHA.
Key factors for a safe MIPD
Many studies have already demonstrated that MIPD is feasible with comparable results with open PD (5,6). The presence of a trained laparoscopic surgeon in the setting of a tertiary center and a careful selection of patients are the most important factors that affect the outcomes of this demanding procedure (30-32). Although few studies concerning this subject have been published, anatomical variations such as aberrancies in the liver vascular supply does not seem to influence the results of MIPD.
Based on current literature and our experience, we developed a list of key factors that should be considered when planning a MIPD in presence of an aberrant RHA arising from the SMA (Table 2).
Table 2
| Accessory or replaced RHA |
| RHA from SMA, CT, GDA or aorta |
| Presence of an accessory or replaced LHA |
| Diameter of the aberrant artery |
| Diameter of an eventual proper RHA |
| Tumor encasement of the artery |
| Distance of the tumor from the aberrant artery |
MIPD, minimally-invasive pancreatoduodenectomy; RHA, right hepatic artery; SMA, superior mesenteric artery; CT, celiack trunk; GDA, gastroduodenal artery.
The first step is distinguishing between an aRHA and a rRHA; the existence of a modal RHA leaves the possibility to the pancreatic surgeon to perform the aRHA ligation without reconstruction with a reduced risk to develop postoperative ischemia-related complications. This reduces the complexity and operative time of the surgical procedure and the risk of a R1 resection margin if a conservative approach is preferred in case of a tumor in contact with the aberrant artery. Contrast-enhanced CT-scan is a widely diffused imaging test that associates rapidity and accuracy in identifying vascular anomalies of the liver arterial supply (33). Apart from discriminating between an aRHA and a rRHA, CT-scan precisely define other anatomical features that can influence the preoperative and intra-operative attitude. Firstly, although aRHA and rRHA most commonly originates from the SMA, aberrant RHAs originating from other vessels such as the gastroduodenal artery, the celiac trunk or the aorta are also documented (12); the preoperative assessment of the arterial anatomy is of mainstay importance as it reduces the risk of an inadvertent vascular injury. Moreover, the anatomical aberrancies involving the left hepatic artery (LHA) are also frequently reported and the coexistence of an anatomical variation of both RHA and LHA can be found in 4–8% of patients (12,34,35). This eventuality represents a pitfall for many reasons: a double aberrancy logically increases the risk to determine a severe liver ischemic damage in case of injury or necessity to perform resection of one or both of liver arteries; a conservative management is of further complexity; a second anatomical aberrancy adds another element whose management must be considered not to compromise the radicality and to guarantee the best oncological results to the patient. On the other hand, an accessory LHA could partially provide for arterial supply compensation in case of aberrant RHA ligation without reconstruction.
Another anatomical main aspect to consider is represented by the caliber of the arteries. When the preoperative imaging and the intraoperative assessment show that the aberrant artery has a little caliber, especially if it is an aRHA, its ligation can be considered less risky for the liver irroration.
If arterial reconstruction is needed in case of a large caliber RHA arising from the SMA to avoid an unduly ischemic injury, laparotomic conversion to perform the anastomosis is suggested.
Finally, one more factor that must be considered when conducting a MIPD is the tumor anatomy. The necessity to perform a ligation and division of a previously identified aberrant RHA often depends on its relationship with the tumor. The more the distance between them is reduced, the more increases the risk of a marginal oncological status in case of a conservative approach with the only study published on this topic setting the cut-off at 10 mm (29). On the other hand, the eventual encasement of the aberrant artery by the tumor can induce the progressive formation of arterial shunts and make its ligation without reconstruction safe.
A flow-chart on the preoperative and intraoperative management of MIPD is proposed (Figure 1).
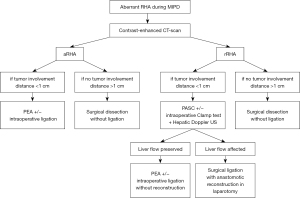
Conclusions
MIPD in case of aberrant RHA originating from the SMA is safe and feasible provided that an adequate anatomical identification of the artery and its relationship with the tumor is performed. Contrast-enhanced CT-scan has satisficing accuracy for this purpose but it lacks the advantages of a dynamic imaging test. More studies are needed to evaluate the benefits of angiography in assessing the contribution of the aberrant artery to the whole liver irroration and in performing an angioembolization to induce shunts opening before vascular ligation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Digestive Medicine Research for the series “Focus on Technical Advancement in Mini-invasive HPB Surgery”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-8/rc
Peer Review File: Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-8/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-8/coif). The series “Focus on Technical Advancement in Mini-invasive HPB Surgery” was commissioned by the editorial office without any funding or sponsorship. ER served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Digestive Medicine Research from September 2020 to August 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. [Crossref] [PubMed]
- Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an Interna-tional Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20-5. [Crossref] [PubMed]
- Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761-8. [Crossref] [PubMed]
- Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017;161:584-91. [Crossref] [PubMed]
- Nickel F, Haney CM, Kowalewski KF, et al. Laparoscopic Versus Open Pancreaticoduodenectomy: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann Surg 2020;271:54-66. [Crossref] [PubMed]
- Aiolfi A, Lombardo F, Bonitta G, et al. Systematic review and updated network meta-analysis comparing open, laparoscopic, and robotic pancreaticoduodenectomy. Updates Surg 2021;73:909-22. [Crossref] [PubMed]
- Nakata K, Higuchi R, Ikenaga N, et al. Precision anatomy for safe approach to pancreatoduodenectomy for both open and minimally invasive procedure: A systematic review. J Hepatobiliary Pancreat Sci 2022;29:99-113. [Crossref] [PubMed]
- Sun R, Yu J, Zhang Y, et al. Perioperative and oncological outcomes following minimally invasive versus open pancreaticoduodenectomy for pancreatic duct adenocarcinoma. Surg Endosc 2021;35:2273-85. [Crossref] [PubMed]
- Ashouri Y, Ho K, Ho H, et al. Minimally invasive vs open pancreatoduodenectomy on oncological adequacy: a propensity score-matched analysis. Surg Endosc 2022; Epub ahead of print. [Crossref] [PubMed]
- Nagakawa Y, Nakata K, Nishino H, et al. International expert consensus on precision anatomy for minimally invasive pancreatoduodenectomy: PAM-HBP surgery project. J Hepatobiliary Pancreat Sci 2022;29:124-35. [Crossref] [PubMed]
- Varotti G, Gondolesi GE, Goldman J, et al. Anatomic variations in right liver living donors. J Am Coll Surg 2004;198:577-82. [Crossref] [PubMed]
- Yan J, Feng H, Wang H, et al. Hepatic artery classification based on three-dimensional CT. Br J Surg 2020;107:906-16. [Crossref] [PubMed]
- Mansour S, Damouny M, Obeid M, et al. Impact of Vascular Anomalies on Pancre-atoduodenectomy Procedure. J Clin Med Res 2021;13:158-63. [Crossref] [PubMed]
- Ishikawa M, Yamagami T, Kakizawa H, et al. Preoperative Coil Embolization in Patients With a Replaced Hepatic Artery Scheduled for Pancreatectomy With Splanchnic Artery Resection Helps to Prevent Ischemic Organ Injury. J Comput Assist Tomogr 2016;40:172-6. [Crossref] [PubMed]
- Cloyd JM, Chandra V, Louie JD, et al. Preoperative embolization of replaced right hepatic artery prior to pancreaticoduodenectomy. J Surg Oncol 2012;106:509-12. [Crossref] [PubMed]
- El Amrani M, Leteurtre E, Sergent G, et al. Pancreatic head carcinoma and right hepatic artery: embolization management-A case report. J Gastrointest Oncol 2014;5:E80-3. [PubMed]
- Marichez A, Turrini O, Fernandez B, et al. Does pre-operative embolization of a replaced right hepatic artery before pancreaticoduodenectomy for pancreatic adenocarcinoma affect postoperative morbidity and R0 resection? A bi-centric French cohort study. HPB (Oxford) 2021;23:1683-91. [Crossref] [PubMed]
- AlMasri S, Paniccia A, Zureikat AH. Robotic Pancreaticoduodenectomy for a Technically Challenging Pancreatic Head Cancer. J Gastrointest Surg 2021;25:1359. [Crossref] [PubMed]
- Mazzola M, Morini L, Crippa J, et al. Totally Laparoscopic Pancreaticoduodenectomy: Technical Notes. Chirurgia (Bucur) 2020;115:385-93. [Crossref] [PubMed]
- Kim JH, Gonzalez-Heredia R, Daskalaki D, et al. Totally replaced right hepatic artery in pancreaticoduodenectomy: is this anatomical condition a contraindication to minimally invasive surgery? HPB (Oxford) 2016;18:580-5. [Crossref] [PubMed]
- Zhang YH, Zhang CW, Hu ZM, et al. Pancreatic cancer: Open or minimally invasive surgery? World J Gastroenterol 2016;22:7301-10. [Crossref] [PubMed]
- Pittau G, Cabus Sanchez S, Laurenzi A, et al. Superior mesenteric artery “first” approach during pancreatoduodenectomy: How we do it laparoscopically. HPB 2016;18:e121-2. [Crossref]
- Ogiso S, Conrad C, Araki K, et al. Posterior approach for laparoscopic pancreaticoduo-denectomy to prevent replaced hepatic artery injury. Ann Surg Oncol 2013;20:3120. [Crossref] [PubMed]
- Nguyen TK, Zenati MS, Boone BA, et al. Robotic pancreaticoduodenectomy in the presence of aberrant or anomalous hepatic arterial anatomy: safety and oncologic outcomes. HPB (Oxford) 2015;17:594-9. [Crossref] [PubMed]
- Wang S, Chen Q, Liu S, et al. The Impact of Aberrant Hepatic Artery on Resection Margin and Outcomes of Laparoscopic Pancreatoduodenectomy: A Single-Center Report. World J Surg 2021;45:3183-90. [Crossref] [PubMed]
- Kim JH, Gonzalez-Heredia R, Daskalaki D, et al. Totally replaced right hepatic artery in pancreaticoduodenectomy: is this anatomical condition a contraindication to minimally invasive surgery? HPB (Oxford) 2016;18:580-5. [Crossref] [PubMed]
- Giani A, Mazzola M, Morini L, et al. Hepatic vascular anomalies during totally laparoscopic pancreaticoduodenectomy: challenging the challenge. Updates Surg 2022;74:583-90. [Crossref] [PubMed]
- Zhang W, Wang K, Liu S, et al. A single-center clinical study of hepatic artery variations in laparoscopic pancreaticoduodenectomy: A retrospective analysis of data from 218 cases. Medicine (Baltimore) 2020;99:e20403. [Crossref] [PubMed]
- Okada K, Kawai M, Hirono S, et al. A replaced right hepatic artery adjacent to pancreatic carcinoma should be divided to obtain R0 resection in pancreaticoduodenectomy. Langenbecks Arch Surg 2015;400:57-65. [Crossref] [PubMed]
- Dokmak S, Aussilhou B, Ftériche FS, et al. The outcome of laparoscopic pancreatoduo-denectomy is improved with patient selection and the learning curve. Surg Endosc 2022;36:2070-80. [Crossref] [PubMed]
- Coppola A, Stauffer JA, Asbun HJ. Laparoscopic pancreatoduodenectomy: current status and future directions. Updates Surg 2016;68:217-24. [Crossref] [PubMed]
- Jones LR, Zwart MJW, Molenaar IQ, et al. Robotic Pancreatoduodenectomy: Patient Se-lection, Volume Criteria, and Training Programs. Scand J Surg 2020;109:29-33. [Crossref] [PubMed]
- Ohgiya Y, Gokan T, Munechika H. Demonstration of aberrant hepatic and gastric arteries with helical CT. Invest Radiol 1999;34:579-84. [Crossref] [PubMed]
- Covey AM, Brody LA, Maluccio MA, et al. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology 2002;224:542-7. [Crossref] [PubMed]
- Suzuki T, Nakayasu A, Kawabe K, et al. Surgical significance of anatomic variations of the hepatic artery. Am J Surg 1971;122:505-12. [Crossref] [PubMed]
Cite this article as: Castaldi A, Chierici A, El Zibawi M, Rosso E, Iannelli A. Management of an aberrant right hepatic artery arising from the superior mesenteric artery during minimally-invasive pancreatoduodenectomy—a narrative review. Dig Med Res 2022;5:34.


