
Gastric adenocarcinoma peritoneal carcinomatosis: a narrative review
Introduction and epidemiology
Each year gastric adenocarcinoma (GA) kills over 750,000 patients globally, ranking fifth in cancer incidence and fourth for mortality (1). Five-year overall survival for all stages is approximately 31% in the USA and 26% in Europe (2). While prognosis is slightly improved in Asia, GA is still the leading cause of cancer death in Asian men (1). Few patients live 5 years past diagnosis of stage IV disease (3). Encouragingly, the overall incidence of GA has been decreasing. This trend has been attributed to multiple factors including improved screening, dietary modifications, and proactive treatment of the carcinogen H. pylori (3). Despite overall decreasing incidence, the absolute number of cases has remained stable, and the incidence may be increasing in younger populations (4).
In this review, we summarize the care of patients with GA and discuss areas of research interest for future management, with particular emphasis on peritoneal metastasis. The peritoneum is a common site of metastasis (5) and the most common site of recurrence (6,7). Radiographically occult peritoneal carcinomatosis (PC) may be found synchronously at diagnosis in up to 40% of patients selected for diagnostic laparoscopy (DL) in a Western cohort (8). Nonetheless, PC may be underdiagnosed due to slow adoption of DL (9). PC can result in a variety of co-morbidities distinct from those associated with solid organ metastases, as well as mortality from mesenteric invasion. Thus, PC from GA origin represents a significant clinical challenge.
The management of peritoneal-only GA metastasis is also unique. Empirically, the clearance of cytologically detectible cancer cells from the abdomen using systemic chemotherapy has been associated with improved disease-specific survival (10). This important finding suggested that the subgroup of patients with PC as their only site of GA metastasis have a natural history separate from those with solid organ metastasis and has generated significant interest in early PC detection and PC-specific therapy. We highlight hyperthermic intraperitoneal chemotherapy (HIPEC), an approved therapy for PC derived from certain abdominal cancers but not GA. Despite its extensive investigation globally, repurposing cytoreductive surgery (CRS) with HIPEC has not been approved for PC from GA in the USA. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-94/rc).
Methods
Search strategy
PubMed and Google Scholar databases were queried for primary articles and reviews in English from 2000 to October, 2021 using the search terms “Gastric Adenocarcinoma Peritoneal Carcinomatosis” (Table S1). Articles were included based on the authors’ discretion.
Carcinomatosis diagnosis and staging
Two management paradigms
Geographic differences in tumor biology and incidence have caused two treatment paradigms to emerge for patients with GA. In Asia, high incidence has led to proactive screening programs that start at age 40 and occur every 2–3 years (11). Screening is performed using endoscopy or, less sensitively, upper GI series. These programs are considered highly successful. For instance, implementation of a national screening program in South Korea has resulted in an increase in detection rate of early-stage GA to greater than 50% of new cases and has dramatically improved prognosis (12).
GA in Western populations, in contrast, is relatively rare. Because of its indolent symptoms and lack of national screening programs, GA in the West presents at a more advanced stage with higher likelihood of synchronous PC, making the “surgery-first” approach that is common in Asian early-stage disease less appropriate. Thus, a higher proportion of Western patients present with synchronous carcinomatosis. Accordingly, in Western patients with T1b disease or greater, DL should be performed to determine the presence of peritoneal disease, and peritoneal lavage fluid should be collected for cytopathology. Once adequate clinical staging has occurred, including solid organ and regional lymph node assessment, further treatment options are assessed (Figure 1). If disease is restricted to the primary site (M0), the patient should be considered for neoadjuvant systemic chemotherapy and gastrectomy with at least 5-cm gross margins. Depending on the histological features and lymph node assessment, adjuvant chemotherapy may be necessary. Radiographic surveillance should occur at regular intervals to monitor for recurrence.
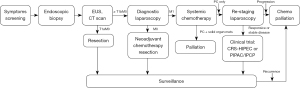
If tumor has spread beyond the primary site (M1), systemic chemotherapy should be employed as primary treatment. The role of surgery for palliation of bleeding and obstruction is generally accepted. However, whether chemotherapy should be used as definitive therapy or as a potential bridge to palliative surgery is dependent on sites of metastasis and response. Metastatic GA to solid organs is typically considered non-curative with surgery. In some centers, PC may be treated with surgery and regional chemotherapy in the setting of a clinical trial. In such cases, PC-only metastasis may be amenable to cytoreduction and gastrectomy if technically feasible and if the burden of disease is stable after neoadjuvant systemic chemotherapy. A post-chemotherapy DL should be performed if CRS is being considered to assess disease progression, and CRS including gastrectomy may be possible. PC-only metastasis that progresses following chemotherapy should not be cytoreduced due to aggressive tumor biology.
Initial workup
Symptoms of low-volume carcinomatosis, like those of primary gastric cancer, can be non-specific and lead to a delay in diagnosis. Overt signs of advanced PC can include abdominal distension with a fluid wave and bowel obstruction. High suspicion is warranted in patients with known germline predisposition to GA, such as hereditary diffuse gastric cancer syndrome (CDH1), familial adenomatous polyposis (APC), hereditary non-polyposis colon cancer [mismatch repair (MMR) genes] Li-Fraumeni syndrome (p53), and Peutz-Jeghers syndrome (STK11/LKB1) (13). Endoscopy should be performed in patients with clinical alarm symptoms and for those who fit high-risk categories. On endoscopy, features concerning for advanced stage disease and carcinomatosis include Borrmann type IV lesions and linitis plastica, indicative of mucosal and submucosal spread that correlates with aggressive biology (14,15).
While computed tomography (CT) scan is sufficient for detecting grossly enlarged lymph node involvement greater than 1 cm, it is not sensitive for determining peritoneal metastasis (16). CT findings specific for PC include omental thickening, presence of ascites, and peritoneal hyperenhancement (17). Problematically, small deposits of tumor are often not distinguishable from adjacent soft tissue (18). Other imaging modalities provide inferior diagnostic capability to CT scan but may be used adjunctively. Fluorodeoxyglucose (FDG)-positron emission tomography (PET) combined with CT is specific but not sensitive to detect occult metastatic disease, especially compared with DL (19). Furthermore, FDG-PET may cause additional delays in initiating systemic chemotherapy (20). The resolution of soft tissue using magnetic resonance imaging (MRI) may be beneficial in detection of PC for appendiceal and colorectal adenocarcinoma (21), however this advantage has not been demonstrated for carcinomatosis of GA origin.
Laparoscopy for detection of carcinomatosis
Due to low radiographic detection and high incidence, the standard for staging the peritoneum is laparoscopic visualization with cytopathologic analysis of peritoneal lavage fluid (see below). In Western cohorts, macroscopic peritoneal deposits are found synchronously in 14–17% of all patients diagnosed with GA of any stage (22,23), and up to 41% of DLs are positive for microscopic cancer cells (24). Both macroscopic and microscopic peritoneal disease represent advanced stage and are considered M1 findings, as their presence correlates with higher recurrence rates following curative-intent gastrectomy (25) (Table 1). Importantly, DL allows for peritoneal carcinomatosis index (PCI) score to be calculated which provides prognostic data, triage for clinical trials, and a standard with which response to neoadjuvant treatment may be followed (26,27). Thus, DL is a critical component of the assessment for PC in staging and treatment in GA, and is both sensitive and specific for radiographically occult carcinomatosis (28). In the West, more widespread adoption of DL for GA has resulted in decreased rates of non-therapeutic laparotomy; curative-intent gastrectomy is now reserved for patients free of macro- and microscopic peritoneal metastasis. In Asia, DL is typically used more selectively. One Japanese series employed DL prospectively in asymptomatic patients based on Borrmann type 3 or 4 histology or radiographically visible lymph nodes, resulting in a change in management for 47% of patients in whom occult carcinomatosis was discovered (29).
Table 1
| Category | Criteria |
|---|---|
| T | |
| T0 | No evidence of tumor |
| Tis | Intraepithelial tumor without invasion into lamina propria |
| T1a | Tumor invades into lamina propria or muscularis mucosa |
| T1b | Tumor invades into submucosa |
| T2 | Tumor invades into muscularis propria |
| T3 | Tumor invades into subserosa |
| T4a | Tumor invades through serosa |
| T4b | Tumor invades into adjacent structures |
| N | |
| N0 | No evidence of regional lymph node metastasis |
| N1 | 1–2 regional lymph node metastases |
| N2 | 3–6 regional lymph node metastases |
| N3a | 7–15 regional lymph node metastases |
| N3b | 16 or more regional lymph node metastases |
| M | |
| M0 | No distant metastasis |
| M1 | Distant metastasis including positive peritoneal cytology |
AJCC, American Joint Committee on Cancer.
Peritoneal lavage
Peritoneal lavage is a useful adjunct to DL that is used to detect the presence of prognostically meaningful microscopic peritoneal tumor cells in patients without macroscopic deposits. During peritoneal lavage, 50–100 cc of saline is instilled into the peritoneum, aspirated, and sent for cytopathologic analysis to determine the presence of adenocarcinoma cells by Papanicolaou stain. This technique is a critical component of a DL, as 13% of patients with macroscopically negative DL will have positive cytology (30). Like patients with macroscopic tumor implants, cytologically-positive patients have demonstrated high rates of recurrence following curative resection (25). Therefore, positive cytology is considered a manifestation of metastatic disease and necessitates systemic chemotherapy. Lavage may miss microscopic peritoneal cancer cells and does not necessarily indicate tumor biology, as 29% of cytologically-negative patients develop a peritoneal recurrence even after R0 resection of their primary tumor (6). However, among cytology-positive patients who undergo systemic chemotherapy, the subcategory of patients for whom repeat staging laparoscopy shows conversion to cytology-negative has been associated with improved disease-free survival (10,31). This finding suggested that small-volume peritoneal-only metastasis may benefit from tumor clearance relative to the broad category of M1-stage patients. Treating microscopic and low volume PC with peritoneal-targeted therapy (see below) may therefore be a rational strategy for this subset of patients with advanced cancer.
Treatment
Systemic chemotherapy
Systemic chemotherapy is the primary treatment option for patients with carcinomatosis. The MAGIC trial changed the treatment paradigm of GA in the West to favor a chemotherapy-first approach by demonstrating an improvement in 5-year OS with perioperative ECF [epirubicin, cisplatin, 5-fluorouracil (5-FU)] from 23% to 36% compared with surgery alone (32). A similar trial observed improved outcomes with perioperative cisplatin plus 5-FU alone (33). The triplet therapy FLOT (5-FU, leucovorin, oxaliplatin, and docetaxel) began in Germany and quickly progressed through trials in the metastatic and perioperative settings, showing efficacy and improved tolerability compared with DCF (docetaxel, cisplatin, fluorouracil) (34,35). the definitive trial was FLOT4, first showing improved pathologic complete response (16% vs. 6%) compared with ECF/ECX [epirubicin, cisplatin, capecitabine (Xeloda)] (36), then improved OS at 1-, 3-, and 5 years (37). Thus, FLOT has become the recommended first-line perioperative treatment for GA in the West. Other treatment combinations in use include XELOX (capecitabine and oxaliplatin) and FOLFOX (5-FU, leucovorin, and oxaliplatin), typically reserved for select patients with contraindication to triplet therapy. Despite this level 1 evidence for FLOT, several issues remain, including applicability beyond Europe and significant toxicity associated with triplet therapy. Additionally, the use of traditional perioperative chemotherapeutic agents in patients with microsatellite-instability (MSI) high tumors may be ineffective (38).
Due to high recurrence rates in the West, adjuvant systemic chemotherapy is recommended in patients who have undergone resection and D2 lymphadenectomy. As previously mentioned, level 1 evidence of FLOT as perioperative systemic chemotherapy demonstrated improved overall survival (37). Additionally, National Comprehensive Cancer Network (NCCN) recommends FOLFOX or XELOX as adjuvant systemic chemotherapy, the latter regimen derived from level 1 evidence in the CLASSIC trial (39). In Asia, adjuvant chemotherapy is employed selectively for cases of advanced stage disease including carcinomatosis. S-1 is used as an adjuvant with or without docetaxel, although evidence has suggested improved survival with the combination adjuvant therapy (40).
Targeted therapy
Unfortunately, systemic therapy for carcinomatosis is rarely curative, and further treatment options are needed. Molecular subclassifications of GA have been developed based on large-scale genomic analysis and offer opportunity to treat advanced-stage disease tailored to the presence of important markers. The Cancer Genome Atlas (TCGA) research network classified four unique molecular categories of GA based on analysis of bulk RNA sequencing from hundreds of primary GA tumors: Epstein-Barr virus (EBV) positive, MSI high, genomically stable (GS), and chromosomal instability (CIN) (41). EBV-positive GA appears to have prominent lymphocytic infiltration and is detected using an in-situ hybridization assay (42). For MSI high tumors, downregulation of MMR genes (e.g., MLH1, MSH2) can lead to signature microsatellite repeats throughout the genome and is associated with tumorigenesis. Tumors lacking EBV infection and MSI were classified by chromosomal count as normal (GS) or possessing a high degree of aneuploidy (CIN). Certain TCGA categories may be susceptible to biologic or targeted therapies depending on their etiology (see below). Notably, other analyses have yielded alterative subclassifications (43).
TCGA classification has utility in selection of targeted therapy for patients with advanced GA including carcinomatosis. Checkpoint blockade immunotherapy should be considered in patients with EBV-positive and MSI high tumors, such as pembrolizumab and nivolumab (44). Chromosomally unstable tumors can be driven by targetable tyrosine kinase mutations including human epidermal growth factor receptor 2 (HER2). The NCCN guidelines therefore recommend molecular testing of the primary tumor for the following: MMR genes, HER2, EBV, and programmed death-ligand 1 (PD-L1). Pembrolizumab (45), bevacizumab (46), apatinib (47), and trastuzumab (48) have all been approved for use in patients with GA selected based on results of these molecular tests. Even with genetic susceptibility, however, none of these agents have been curative for any molecular classification. One possible explanation for resistance is mutational diversity, as there exists a large degree of primary-metastasis and intratumor heterogeneity (49).
Gastrectomy
The presence of carcinomatosis is considered a contraindication to curative-intent surgical management of GA. However, it is important to understand surgical principles that guide management for patients without carcinomatosis or who are eligible for clinical trials (described below). For early-stage gastric tumors, endoscopic mucosal resection (EMR) is used primarily in endemic areas including Asia where screening programs detect early-stage GA. In contrast, the majority of tumors discovered in Western populations are greater than T1 at diagnosis or have diffuse-type histology that can spread submucosally, making EMR less applicable. Additionally, almost half of T2 tumors have pathologically positive lymph nodes, making EMR inadequate for complete pathologic staging for most GA in Western patients (50). Thus, an anatomic partial or total gastrectomy is the most common operation for eligible patients in the Western GA population. Distal gastrectomy with D2 lymphadenectomy may be performed laparoscopically with equivalent 5-year survival as the open procedure (51).
Palliation
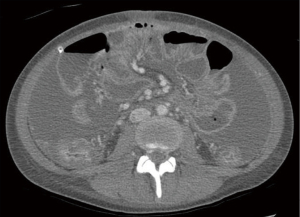
Unfortunately, nearly all patients with carcinomatosis from GA will have disease progression. Patterns of recurrence involve peritoneum, liver, lung, bone, and brain. A multidisciplinary group is best suited to manage disease recurrences. Of central importance is a comprehensive end of life discussion including palliative contingencies for when recurrence and complications arise. For patients with carcinomatosis, recurrence in the abdomen can cause both large and small bowel obstruction leading to compressive symptoms and possible perforation. Enteric tubes can be critical in managing symptoms and serve as a valuable bridge to renourish patients in preparation for palliative treatment (52). Ascites can cause pain and compression, and careful drainage should be considered to provide pain relief. In its final stages, carcinomatosis can manifest as tumor growth into the root of the mesentery (Figure 2). This is considered a pre-terminal finding in the abdomen and can be associated with severe pain. A liberal pain management strategy is appropriate.
Future directions
Intraperitoneal chemotherapy
Intraperitoneal chemotherapy treatments are not considered standard for PC from GA at this time. Nonetheless, they are the topic of intense research efforts for two reasons: (I) the peritoneum is an early, and common, site of metastasis in gastric cancer, and (II) CRS-HIPEC has been approved for peritoneal surface metastasis for tumors of other primary sites (53-55). The intraperitoneal chemotherapy strategies may be categorized as HIPEC, pressurized intraperitoneal aerosol chemotherapy (PIPAC), and intraperitoneal chemotherapy port (IPCP). Below we consider the evidence for each treatment modality.
HIPEC
Protocols for CRS-HIPEC follow the same general sequence after neoadjuvant chemotherapy is completed. Operative exploration is performed, starting with resection of the primary tumor, modified D2 lymphadenectomy, and metastasectomy with peritonectomy of all macroscopic disease. Depending on extent of invasion, a multi-visceral resection may be necessary in certain cases to remove all evidence of disease. Reconstruction is performed to restore enteric continuity. The abdomen is closed over large-bore catheters, sodium thiosulfate is infused for renal protection, and chemotherapy diluted into dialysis solution is administered via perfusion circuit to the peritoneal cavity. Perfusion duration and chemotherapeutic agents vary based on institutional protocols, and no single protocol or agents have proven to have superior efficacy over the others.
Early trials in Asia suggested that CRS-HIPEC improved survival relative to control patients that had surgery alone (56-58), encouraging international interest in replicating these findings in other populations. Meta-analysis of twenty prospective trials published between 1987 and 2011 demonstrated improved 1-, 2-, and 3-year OS, but no difference in OS at 5 years (59). Other phase II trials have shown CRS-HIPEC to correlate with achieving the upper limit of OS in stage IV disease (60,61). While potentially encouraging, interpretation of these data is difficult due to small sample sizes and significant practice changes within that timeframe. To address the former, larger and more recent analyses have been performed on nationwide datasets. The CYTO-CHIP (n=277) in France, DGAV (n=315) in Germany, SICO (n=91) in Italy, and GECOP (n=88) in Spain have all demonstrated efficacy and suggested improved OS in patients with PC from GA selected based on low PCI (62-65).
There is a need to integrate prospective HIPEC trials within the context of modern treatment algorithms including perioperative FLOT chemotherapy and routine DL-based staging. Recent reports have included these evidence-based practices and are more reflective of outcomes of modern patients. The forthcoming report of the GASTRIPEC-I trial from Germany comparing CRS-HIPEC to CRS alone is greatly anticipated, as improvement in DFS but not OS was reportedly observed (66). In the USA, two single arm trials of CRS-HIPEC are ongoing (61,67). In Europe, the phase III RCTs GASTRICHIP in France and PREVENT in Germany both randomized patients between CRS and CRS-HIPEC (68,69). Finally, neoadjuvant laparoscopic HIPEC has also been shown to be feasible therapeutic option prior to CRS (70).
Non-HIPEC abdominal chemotherapy
For patients with disease not amenable to cytoreduction and/or a PCI score exclusive of HIPEC protocols, several investigational treatment modalities are being studied as either neoadjuvant or definitive/palliative therapy. In PIPAC, laparoscopically-delivered aerosolized chemotherapy insufflates the abdomen achieving high pressures and tissue permeability (71,72). Typically, mitomycin C and cisplatin are employed intra-abdominally, and most protocols combine PIPAC with concurrent systemic chemotherapy. The technique is safe and potentially cost-effective (73,74). Early survival estimates from small trials of PIPAC plus chemotherapy have shown efficacy in comparison to chemotherapy alone (75), as well as responses conferring eligibility for CRS-HIPEC trials (76). Larger-scale trials are underway (71,77).
In IPCP, patients with known unresectable disease undergo a laparoscopic procedure to position an intraabdominal catheter, and an access port is secured in the subcutaneous tissue. Unlike laparoscopic HIPEC and PIPAC, this arrangement allows patients to receive outpatient systemic and intraperitoneal chemotherapy (i.e., bidirectional chemotherapy) without further need for anesthesia. Outcomes for IPCP were favorable (78,79), however the PHOENIX-GC trial failed to demonstrate superiority of bidirectional chemotherapy versus systemic chemotherapy alone (80). Nonetheless, there is still interest due to the theoretical benefits of outpatient bidirectional therapy, and trials outside of Asia are ongoing (81,82).
Liquid biopsy
Prognostic information from peripheral blood (i.e., liquid biopsy) has long been the subject of research interest. Circulating tumor DNA (ctDNA) detected by next-generation sequencing has been linked to stage (83) and peritoneal recurrence (84) in GA. Clinically actionable mutations are also detectible by ctDNA (85), although there is significant primary-metastatic inter-tumor heterogeneity in GA which may be potentially confounding (49). Additionally, liquid biopsy may have utility in surveillance, as post-gastrectomy recurrence can be predicted by ctDNA (86). The NCCN guidelines assess liquid biopsy as potentially useful in patients with advanced disease who are unable to undergo biopsy, given the precaution that false negatives are possible.
Future directions of liquid biopsy may surpass data gathered from ctDNA. Recently, the transcriptomic landscape of GA cells within ascites fluid was elucidated at the single-cell level (87). The two distinct cancer cell states, gastric-dominant and GI-mixed, had differential prognoses, and a 12-gene expression signature was demonstrated to be predictive of subtype. With enhanced capabilities to observe single-cell states at the transcriptome level and spatial resolution, further exciting biological insights are anticipated.
Conclusions
As a disease that has been associated with high incidence and limited therapeutic options for decades, GA treatment has advanced significantly in the last 20 years on all fronts—prevention, diagnosis, staging, targeted therapy, and perioperative chemotherapy. Despite these improvements, GA remains a cause of high mortality worldwide, and new therapies are urgently needed to prolong survival. Such rapid advancement may be self-limiting, as a significant challenge currently lies in reconciling historical knowledge with new treatment standards. For example, most HIPEC protocols historically included ECF, not FLOT, as standard perioperative systemic chemotherapy regimens. Survival advantage seen in patients with cytological conversion was likewise not performed with modern chemotherapy agents. Are these past observations reproducible and/or still relevant using today’s therapies? Modern trials aim to resolve these discrepancies. Clinically, patients with PC have unique disease biology that is separate from solid organ metastasis, yet there remain few treatment options. A significant limitation in the existing evidence on intraperitoneal chemotherapy reviewed here is its low quality, with mostly retrospective analysis and small trials owing to complex therapeutic algorithms and relative rarity of disease. As the efficacy of intraperitoneal chemotherapy becomes clarified, patients suffering with PC may have alternative methods of disease control on the horizon.
Acknowledgments
Funding: This research was supported in part by the Intramural Research Program, National Cancer Institute, National Institutes of Health.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Andrew M. Blakely and Oliver S. Eng) for the series “Peritoneal Carcinomatosis: History and Future” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-94/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-94/coif). The series “Peritoneal Carcinomatosis: History and Future” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol 2019;14:26-38. [Crossref] [PubMed]
- Ajani JA, Lee J, Sano T, et al. Gastric adenocarcinoma. Nat Rev Dis Primers 2017;3:17036. [Crossref] [PubMed]
- Arnold M, Park JY, Camargo MC, et al. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 2020;69:823-9. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Sundquist K, et al. Metastatic spread in patients with gastric cancer. Oncotarget 2016;7:52307-16. [Crossref] [PubMed]
- D'Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 2004;240:808-16. [Crossref] [PubMed]
- Ikoma N, Chen HC, Wang X, et al. Patterns of Initial Recurrence in Gastric Adenocarcinoma in the Era of Preoperative Therapy. Ann Surg Oncol 2017;24:2679-87. [Crossref] [PubMed]
- Burke EC, Karpeh MS, Conlon KC, et al. Laparoscopy in the management of gastric adenocarcinoma. Ann Surg 1997;225:262-7. [Crossref] [PubMed]
- Groh EM, Gupta S, Brown ZJ, et al. Staging Laparoscopy is Underutilized in the Management of Gastric Adenocarcinoma. Ann Surg Oncol 2020;27:1473-9. [Crossref] [PubMed]
- Mezhir JJ, Shah MA, Jacks LM, et al. Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol 2010;17:3173-80. [Crossref] [PubMed]
- Hamashima C, Goto R. Potential capacity of endoscopic screening for gastric cancer in Japan. Cancer Sci 2017;108:101-7. [Crossref] [PubMed]
- Suh YS, Yang HK. Screening and Early Detection of Gastric Cancer: East Versus West. Surg Clin North Am 2015;95:1053-66. [Crossref] [PubMed]
- Lott PC, Carvajal-Carmona LG. Resolving gastric cancer aetiology: an update in genetic predisposition. Lancet Gastroenterol Hepatol 2018;3:874-83. [Crossref] [PubMed]
- Jung K, Park MI, Kim SE, et al. Borrmann Type 4 Advanced Gastric Cancer: Focus on the Development of Scirrhous Gastric Cancer. Clin Endosc 2016;49:336-45. [Crossref] [PubMed]
- Ikoma N, Agnes A, Chen HC, et al. Linitis Plastica: a Distinct Type of Gastric Cancer. J Gastrointest Surg 2020;24:1018-25. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286-312. [Crossref] [PubMed]
- Kim SJ, Kim HH, Kim YH, et al. Peritoneal metastasis: detection with 16- or 64-detector row CT in patients undergoing surgery for gastric cancer. Radiology 2009;253:407-15. [Crossref] [PubMed]
- Guo JC, Chang CC, Yang CY, et al. Computed tomographic characteristics for patients with unresectable gastric cancer harboring low-volume peritoneal carcinomatosis. Med Oncol 2017;34:143. [Crossref] [PubMed]
- Smyth E, Schöder H, Strong VE, et al. A prospective evaluation of the utility of 2-deoxy-2-[(18) F]fluoro-D-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer. Cancer 2012;118:5481-8. [Crossref] [PubMed]
- Gertsen EC, Borggreve AS, Brenkman HJF, et al. Evaluation of the Implementation of FDG-PET/CT and Staging Laparoscopy for Gastric Cancer in The Netherlands. Ann Surg Oncol 2021;28:2384-93. [Crossref] [PubMed]
- Low RN, Barone RM. Imaging for Peritoneal Metastases. Surg Oncol Clin N Am 2018;27:425-42. [Crossref] [PubMed]
- Thomassen I, van Gestel YR, van Ramshorst B, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer 2014;134:622-8. [Crossref] [PubMed]
- Gretschel S, Siegel R, Estévez-Schwarz L, et al. Surgical strategies for gastric cancer with synchronous peritoneal carcinomatosis. Br J Surg 2006;93:1530-5. [Crossref] [PubMed]
- Ribeiro U Jr, Gama-Rodrigues JJ, Safatle-Ribeiro AV, et al. Prognostic significance of intraperitoneal free cancer cells obtained by laparoscopic peritoneal lavage in patients with gastric cancer. J Gastrointest Surg 1998;2:244-9. [Crossref] [PubMed]
- Bentrem D, Wilton A, Mazumdar M, et al. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol 2005;12:347-53. [Crossref] [PubMed]
- Yarema R, Ohorchak M, Hyrya P, et al. Gastric cancer with peritoneal metastases: Efficiency of standard treatment methods. World J Gastrointest Oncol 2020;12:569-81. [Crossref] [PubMed]
- Cardona K, Zhou Q, Gönen M, et al. Role of repeat staging laparoscopy in locoregionally advanced gastric or gastroesophageal cancer after neoadjuvant therapy. Ann Surg Oncol 2013;20:548-54. [Crossref] [PubMed]
- Ramos RF, Scalon FM, Scalon MM, et al. Staging laparoscopy in gastric cancer to detect peritoneal metastases: A systematic review and meta-analysis. Eur J Surg Oncol 2016;42:1315-21. [Crossref] [PubMed]
- Irino T, Sano T, Hiki N, et al. Diagnostic staging laparoscopy in gastric cancer: a prospective cohort at a cancer institute in Japan. Surg Endosc 2018;32:268-75. [Crossref] [PubMed]
- Ikoma N, Blum M, Chiang YJ, et al. Yield of Staging Laparoscopy and Lavage Cytology for Radiologically Occult Peritoneal Carcinomatosis of Gastric Cancer. Ann Surg Oncol 2016;23:4332-7. [Crossref] [PubMed]
- Jamel S, Markar SR, Malietzis G, et al. Prognostic significance of peritoneal lavage cytology in staging gastric cancer: systematic review and meta-analysis. Gastric Cancer 2018;21:10-8. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Al-Batran SE, Hartmann JT, Hofheinz R, et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2008;19:1882-7. [Crossref] [PubMed]
- Schulz C, Kullmann F, Kunzmann V, et al. NeoFLOT: Multicenter phase II study of perioperative chemotherapy in resectable adenocarcinoma of the gastroesophageal junction or gastric adenocarcinoma-Very good response predominantly in patients with intestinal type tumors. Int J Cancer 2015;137:678-85. [Crossref] [PubMed]
- Al-Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol 2016;17:1697-708. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948-57. [Crossref] [PubMed]
- Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol 2017;3:1197-203. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Yoshida K, Kodera Y, Kochi M, et al. Addition of Docetaxel to Oral Fluoropyrimidine Improves Efficacy in Patients With Stage III Gastric Cancer: Interim Analysis of JACCRO GC-07, a Randomized Controlled Trial. J Clin Oncol 2019;37:1296-304. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Fukayama M, Ushiku T. Epstein-Barr virus-associated gastric carcinoma. Pathol Res Pract 2011;207:529-37. [Crossref] [PubMed]
- Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449-56. [Crossref] [PubMed]
- Mazloom A, Ghalehsari N, Gazivoda V, et al. Role of Immune Checkpoint Inhibitors in Gastrointestinal Malignancies. J Clin Med 2020;9:2533. [Crossref] [PubMed]
- Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392:123-33. [Crossref] [PubMed]
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Pectasides E, Stachler MD, Derks S, et al. Genomic Heterogeneity as a Barrier to Precision Medicine in Gastroesophageal Adenocarcinoma. Cancer Discov 2018;8:37-48. [Crossref] [PubMed]
- Ikoma N, Blum M, Chiang YJ, et al. Race Is a Risk for Lymph Node Metastasis in Patients With Gastric Cancer. Ann Surg Oncol 2017;24:960-5. [Crossref] [PubMed]
- Huang C, Liu H, Hu Y, et al. Laparoscopic vs Open Distal Gastrectomy for Locally Advanced Gastric Cancer: Five-Year Outcomes From the CLASS-01 Randomized Clinical Trial. JAMA Surg 2022;157:9-17. [Crossref] [PubMed]
- Blumenthaler AN, Ikoma N, Blum M, et al. Relationship between initial management strategy and survival in patients with gastric outlet obstruction due to gastric cancer. J Surg Oncol 2020;122:1373-82. [Crossref] [PubMed]
- Alexander HR Jr, Bartlett DL, Pingpank JF, et al. Treatment factors associated with long-term survival after cytoreductive surgery and regional chemotherapy for patients with malignant peritoneal mesothelioma. Surgery 2013;153:779-86. [Crossref] [PubMed]
- Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006;354:34-43. [Crossref] [PubMed]
- Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012;30:2449-56. [Crossref] [PubMed]
- Fujimura T, Yonemura Y, Muraoka K, et al. Continuous hyperthermic peritoneal perfusion for the prevention of peritoneal recurrence of gastric cancer: randomized controlled study. World J Surg 1994;18:150-5. [Crossref] [PubMed]
- Fujimoto S, Takahashi M, Mutou T, et al. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer 1999;85:529-34. [Crossref] [PubMed]
- Yonemura Y, de Aretxabala X, Fujimura T, et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterology 2001;48:1776-82. [PubMed]
- Coccolini F, Cotte E, Glehen O, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol 2014;40:12-26. [Crossref] [PubMed]
- Rudloff U, Langan RC, Mullinax JE, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol 2014;110:275-84. [Crossref] [PubMed]
- Badgwell B, Ikoma N, Murphy MB, et al. A Phase II Trial of Cytoreduction, Gastrectomy, and Hyperthermic Intraperitoneal Perfusion with Chemotherapy for Patients with Gastric Cancer and Carcinomatosis or Positive Cytology. Ann Surg Oncol 2021;28:258-64. [Crossref] [PubMed]
- Bonnot PE, Piessen G, Kepenekian V, et al. Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J Clin Oncol 2019;37:2028-40. [Crossref] [PubMed]
- Rau B, Brandl A, Piso P, et al. Peritoneal metastasis in gastric cancer: results from the German database. Gastric Cancer 2020;23:11-22. [Crossref] [PubMed]
- Manzanedo I, Pereira F, Rihuete Caro C, et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Gastric Cancer with Peritoneal Carcinomatosis: Multicenter Study of Spanish Group of Peritoneal Oncologic Surgery (GECOP). Ann Surg Oncol 2019;26:2615-21. [Crossref] [PubMed]
- Marano L, Marrelli D, Sammartino P, et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Synchronous Peritoneal Metastases: Multicenter Study of 'Italian Peritoneal Surface Malignancies Oncoteam-S.I.C.O.' Ann Surg Oncol 2021;28:9060-70. [Crossref] [PubMed]
- Rau B, Lang H, Königsrainer A, et al. The effect of hyperthermic intraperitoneal chemotherapy (HIPEC) upon cytoreductive surgery (CRS) in gastric cancer (GC) with synchronous peritoneal metastasis (PM): A randomized multicentre phase III trial (GASTRIPEC-I-trial). Ann Oncol 2021;32:abstr 1376O.
- Brown ZJ, Hernandez JM, Ripley RT, et al. Heated intraperitoneal chemotherapy and gastrectomy for gastric cancer in the U.S.: the time is now. J Gastrointest Oncol 2017;8:1109-13. [Crossref] [PubMed]
- Götze TO, Piso P, Lorenzen S, et al. Preventive HIPEC in combination with perioperative FLOT versus FLOT alone for resectable diffuse type gastric and gastroesophageal junction type II/III adenocarcinoma - the phase III "PREVENT"- (FLOT9) trial of the AIO /CAOGI /ACO. BMC Cancer 2021;21:1158. [Crossref] [PubMed]
- Glehen O, Passot G, Villeneuve L, et al. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC Cancer 2014;14:183. [Crossref] [PubMed]
- Blumenthaler AN, Allen CJ, Ikoma N, et al. Laparoscopic HIPEC for Low-Volume Peritoneal Metastasis in Gastric and Gastroesophageal Adenocarcinoma. Ann Surg Oncol 2020;27:5047-56. [Crossref] [PubMed]
- Raoof M, Malhotra G, Kohut A, et al. PIPAC for the Treatment of Gynecologic and Gastrointestinal Peritoneal Metastases: Technical and Logistic Considerations of a Phase 1 Trial. Ann Surg Oncol 2022;29:175-85. [Crossref] [PubMed]
- Garg PK, Jara M, Alberto M, et al. The role of Pressurized IntraPeritoneal Aerosol Chemotherapy in the management of gastric cancer: A systematic review. Pleura Peritoneum 2019;4:20180127. [Crossref] [PubMed]
- Javanbakht M, Mashayekhi A, Branagan-Harris M, et al. Cost-effectiveness analysis of pressurized intraperitoneal aerosol chemotherapy (PIPAC) in patients with gastric cancer and peritoneal metastasis. Eur J Surg Oncol 2022;48:188-96. [Crossref] [PubMed]
- Struller F, Horvath P, Solass W, et al. Pressurized intraperitoneal aerosol chemotherapy with low-dose cisplatin and doxorubicin (PIPAC C/D) in patients with gastric cancer and peritoneal metastasis: a phase II study. Ther Adv Med Oncol 2019;11:1758835919846402. [Crossref] [PubMed]
- Tidadini F, Abba J, Quesada JL, et al. Effect of Pressurized Intraperitoneal Aerosol Chemotherapy on the Survival Rate of Patients with Peritoneal Carcinomatosis of Gastric Origin. J Gastrointest Cancer 2021; [Epub ahead of print]. [Crossref] [PubMed]
- Sindayigaya R, Dogan C, Demtröder CR, et al. Clinical Outcome for Patients Managed with Low-Dose Cisplatin and Doxorubicin Delivered as Pressurized Intraperitoneal Aerosol Chemotherapy for Unresectable Peritoneal Metastases of Gastric Cancer. Ann Surg Oncol 2022;29:112-23. [Crossref] [PubMed]
- Oliver Goetze T, Al-Batran SE, Pabst U, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in combination with standard of care chemotherapy in primarily untreated chemo naïve upper gi-adenocarcinomas with peritoneal seeding - a phase II/III trial of the AIO/CAOGI/ACO. Pleura Peritoneum 2018;3:20180113. [Crossref] [PubMed]
- Yamaguchi H, Kitayama J, Ishigami H, et al. Breakthrough therapy for peritoneal carcinomatosis of gastric cancer: Intraperitoneal chemotherapy with taxanes. World J Gastrointest Oncol 2015;7:285-91. [Crossref] [PubMed]
- Chan DY, Syn NL, Yap R, et al. Conversion Surgery Post-Intraperitoneal Paclitaxel and Systemic Chemotherapy for Gastric Cancer Carcinomatosis Peritonei. Are We Ready? J Gastrointest Surg 2017;21:425-33. [Crossref] [PubMed]
- Ishigami H, Fujiwara Y, Fukushima R, et al. Phase III Trial Comparing Intraperitoneal and Intravenous Paclitaxel Plus S-1 Versus Cisplatin Plus S-1 in Patients With Gastric Cancer With Peritoneal Metastasis: PHOENIX-GC Trial. J Clin Oncol 2018;36:1922-9. [Crossref] [PubMed]
- Martin SP, Drake JA, Hernandez JM, et al. Bidirectional chemotherapy in patients with gastric cancer and peritoneal metastasis. J Gastrointest Oncol 2020;11:108-11. [Crossref] [PubMed]
- Vatandoust S, Bright T, Roy AC, et al. Phase I open-label trial of intraperitoneal paclitaxel in combination with intravenous cisplatin and oral capecitabine in patients with advanced gastric cancer and peritoneal metastases (IPGP study): study protocol. BMJ Open 2019;9:e026732. [Crossref] [PubMed]
- Hamakawa T, Kukita Y, Kurokawa Y, et al. Monitoring gastric cancer progression with circulating tumour DNA. Br J Cancer 2015;112:352-6. [Crossref] [PubMed]
- Fang WL, Lan YT, Huang KH, et al. Clinical significance of circulating plasma DNA in gastric cancer. Int J Cancer 2016;138:2974-83. [Crossref] [PubMed]
- Shu Y, Wu X, Tong X, et al. Circulating Tumor DNA Mutation Profiling by Targeted Next Generation Sequencing Provides Guidance for Personalized Treatments in Multiple Cancer Types. Sci Rep 2017;7:583. [Crossref] [PubMed]
- Leal A, van Grieken NCT, Palsgrove DN, et al. White blood cell and cell-free DNA analyses for detection of residual disease in gastric cancer. Nat Commun 2020;11:525. [Crossref] [PubMed]
- Wang R, Dang M, Harada K, et al. Single-cell dissection of intratumoral heterogeneity and lineage diversity in metastatic gastric adenocarcinoma. Nat Med 2021;27:141-51. [Crossref] [PubMed]
Cite this article as: Green BL, Davis JL. Gastric adenocarcinoma peritoneal carcinomatosis: a narrative review. Dig Med Res 2022;5:37.


