
How do we assess analgesia efficacy?—a narrative review
Introduction
It is often difficult to accurately assess the severity of pain and its response to treatment, as pain is complex, multi-faceted and has a subjective element. Post-operative pain is very common, and if it is poorly controlled it can have a negative impact on patient outcomes and increase morbidity e.g., with the development of post-operative pulmonary complications. A combination of thorough clinical assessment and the use of appropriate pain measurement tools are vital in eliciting both the intensity of pain and the response to analgesic interventions, in order to optimise patient experience and symptom control. There are many clinical trials that examine the various pain assessment tools, but it is difficult to find a consensus on which to use due to heterogeneity in outcome measures. Additionally the data included from the individual studies is often a hybrid of qualitative and quantitative data, requiring separate statistical analysis. We discuss the tools that we us to assess analgesia efficacy in this narrative review.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-93/rc).
Methods
A search was carried out using MEDLINE-PubMed and Google Scholar looking at acute and chronic pain assessment, as well as that in elderly and paediatric populations. Search terms used were: “acute pain assessment”; “post-operative pain assessment”; “chronic pain assessment”; “neuropathic pain assessment”; “cancer pain”; “pain assessment in dementia”; and “paediatric pain assessment”. Depression, anxiety and pain catastrophizing (the tendency to ruminate on and magnify pain), were also included as they are important additional aspects in pain assessment. The literature reviewed was from between 1975 and July 2021. The articles included are mainly small prospective studies, which consist of those outlining the development of pain assessment tools as well as those critically evaluating them. However, there are also studies based on data from randomised control trials, as well as systematic reviews. As this is a narrative review article, the quality of the evidence has not been graded. See Table 1 for a summary.
Table 1
| Items | Specification |
|---|---|
| Date of search | 10/09/21 |
| Databases and other sources searched | MEDLINE-PubMed, Google Scholar |
| Search terms used | “Acute pain assessment”; “chronic pain” assessment, post-operative pain assessment”; “neuropathic pain assessment”; “cancer pain”; “pain assessment in dementia”; “paediatric pain assessment”; depression, anxiety and pain catastrophizing |
| Timeframe | 2 months |
| Inclusion and exclusion criteria | Articles in English only |
| Selection process | Conducted by Dr. Parrott, content reviewed by Dr. Kelliher |
| Any additional considerations | None |
Discussion
Acute pain
Acute pain and its response to analgesia can be evaluated using a combination of clinical examination and simple assessment scales. Clinical indicators of pain include tachycardia, tachypnoea and hypertension. These are simple to measure and in addition may provide insight into both the severity of pain and the response to/efficacy of analgesia. However, an individual’s experience of pain is more complex, comprising physical and emotional elements. Assessing and treating pain requires more than simply monitoring changes in basic physical parameters and as such a number of tools have been developed. The ‘gold-standard’ is considered to be the visual analogue scale (VAS). This technique consists of a 100 mm unmarked line labelled with ‘no pain’ on the left and ‘worst pain imaginable’ on the right, on which the patient then puts a mark to indicate their level of pain. It requires some explanation to the patient as well as a level of comprehension, which limits its use in some settings such as paediatrics, learning difficulties, dementia and also in the initial post-anaesthetic period when cognition may be impaired (1). An alternative might be the numeric rating scale (NRS) which asks patients to rate their pain on an 11-point scale from 0—‘no pain’ to 10—‘worst pain’, and may be preferred due to its administration simplicity and reliability. However, both tools are straightforward to administer and are reliable, valid and sensitive to change in the measurement of severity of pain, giving an indication of response to treatment (2).
Another simple tool used to assess pain severity is the categorical verbal rating scale (VRS), which uses words such as ‘none’, ‘mild’, ‘moderate’ and ‘severe’ to describe the magnitude of pain. It has high validity with respect to pain intensity, however studies have shown that it is less sensitive than both VAS and NRS (2,3). Although generally quite intuitive to use, the VRS may be limited by language barriers and be difficult to administer in those less able to communicate. VRS have been used to assess changes in both pain intensity (i.e., as measured by changes in pain intensity ratings from baseline to each post-medication assessment) and pain relief, with research indicating that these are related to but distinct from one another. This has implications for clinical trials when considering methods of assessing the impact of pain management tools and analgesia (3).
In patient populations where understanding or communication may be impaired (e.g., in paediatrics or those with dementia) it may be necessary to use alternative pain scales to evaluate acute pain. A common example is the facial pain scale (see Figure 1) many versions of which exist. The Faces Pain Scale-Revised (FPS-R) is one such tool; it consists of 6 gender-neutral line drawings of faces portraying increasing levels of distress, which are scored from 1–10. The patient is asked to indicate which face best represents the level of pain or “hurt” they are feeling at that time (4). Pain assessment in special circumstances is discussed in more detail below.
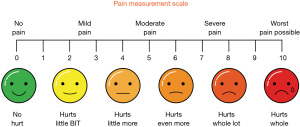
The majority of acute pain as assessed above tends to be somatic (due to visceral/musculoskeletal injury), however neuropathic pain (due to nerve injury/disease) has been shown to be present in the early post-operative period in up to 3% of patients (5), with a higher incidence in certain types of surgery (e.g., hernia repair). As such it is an important consideration when managing those with acute pain as well as chronic. Methods for assessing neuropathic pain are discussed below.
Chronic pain
Chronic pain is complex, and has multiple variables and components that need to be assessed in order to build an accurate picture of the patient’s condition and the response to treatment. The unidimensional scales mentioned above are useful for rapid assessment of current pain intensity and may be used in chronic pain, however it is important to consider that the information gathered may vary depending upon the contextual details of the question. For example, what is the pain at rest vs. with movement, current vs. average, location of the pain etc. As such it may be that these scales may not represent the full picture and should be supplemented by more in-depth tools in order to provide a more accurate picture of chronic pain (6). There are various multi-dimensional scales, functional assessments and psychological tools that have been designed to accompany clinical observation in these patients.
The McGill Pain Questionnaire (MPQ) is a multi-dimensional self-rating tool that produces quantitative data regarding the subjective pain experience, allowing for statistical analysis of treatment efficacy. It consists of 3 major word descriptors for the components of pain: sensory, affective and evaluative. These subgroups, along with a miscellaneous category, have a list of words with a given ranking that the patient must choose from. The sum of the ranked scores produces the pain rating index (PRI). The other key measures are the number of words chosen by the patient, and the Present Pain Intensity determined by a 5-point scale (1-5) (7). This tool and its less time consuming short form (SF-MPQ-2) have been widely used due to their reproducibility, reliability and validity in a diverse population of patients with chronic pain, and can be applied effectively in a number of different languages (6). However, systematic reviews suggest that further high-quality studies to assess retest reliability, cross-cultural validity and measurement error indices (e.g., standard error of measurement) are required, particularly in those with musculoskeletal conditions (8,9).
The brief pain inventory (BPI), also available as a short form (BPI-SF), is another multi-dimensional tool used in the assessment of pain severity and its impact on daily functions, as well as response to treatment. Although originally designed for patients with cancer related pain, it can be used in both chronic and acute pain conditions. It does however take 5–15 minutes to complete and as such is less desirable as a method of repeatedly reassessing response to treatment in the acute pain setting e.g., in the post-operative period. It consists of a diagram of the human form on which patients are asked to shade the affected area, plus 11 different NRS covering pain intensity (present, least, worst and average) and the effect of the pain on their ability to carry out daily activities. It has a good validity and reliability across a number of different cultures and languages, and has been adapted for use in many countries for clinical pain assessment, epidemiological studies and in studies on the effectiveness of pain treatment (10). A recent systematic review into use of the BPI-SF in patients with musculoskeletal conditions found high quality evidence for its validity and responsiveness, though as with the SF-MPQ-2 above there are insufficient reports on its cross-cultural validity and measurement error indices (9).
Functional assessment of pain involves looking into pain related disability, which has been defined as “the extent to which chronic pain interferes with a person’s ability to engage in life activities” (11). This acknowledges the complex interplay between pain intensity, extent and duration, and other factors such as emotional and cognitive responses to this pain. The Pain Disability Index was developed to allow assessment of general and specific aspects of this disability across seven different activities. These are assessed using an 11-point NRS and a general score is generated by the summation of these results. Areas identified include family/home responsibilities, recreation, social activity, occupation, sexual behaviour, self-care, and life support activity. It has been used in clinical and research settings and has good validity, though may have only modest reliability, and as such should be used in conjunction with other physical, systematic and psychological assessments (12).
There are other tools in use that look at specific chronic pain conditions, such as the Rowland-Morris disability questionnaire (RDQ) and the Oswestry disability index (ODI), which assess lower back pain. The ODI is one of the most commonly used tools Internationally. It asks patients to rate their level of disability (0—lack of disability to 5—severe disability) in relation to 10 areas associated with lower back pain, including pain intensity, ability to walk/stand/sit, sleep quality and sexual function. It is a reliable tool that has been widely used in a range of conditions causing lower back pain, particularly lumbar spine stenosis. The RDQ is quick, simple and easy to administer, it asks patients to indicate which of 24 statements concerning physical functions apply to them that day as a result of their lower back pain, giving a score between 0 (no disability) and 24 (maximal disability). It has good scientific validity for use in lower back pain, is used in 12 different languages and a study found that there were fewer reports of ambiguity/incomplete responses when compared to the ODI (13).
Functional pain scores have also been developed for other conditions associated with chronic pain, such as the Majeed pelvic score (MPS) for pelvic fractures. It consists of five subscales including pain, work, sitting, sexual intercourse and standing (sub-divided into walking aids, unaided gait, and walking distance). These are graded by the patient to give a score from 0 to 100 (clinical grade: poor <55, fair 55–69, good 70–84, excellent ≥85), where higher scores represent the best outcomes (14). Although widely used to assess patient function following pelvic fractures, a recent Systematic Review observed poor accuracy and notable inconsistency in the use and reporting of the MPS in the literature. They indicated that interpretation and comparison of research reporting this score should be done cautiously, and that future studies should include detailed accounts of how it has been calculated to allow for verification of presented results and conclusions (15).
Special circumstances
Neuropathic pain
Neuropathic pain is a disease of the central and/or peripheral somatosensory nervous system that can be resistant to treatment and have a significant impact on quality of life. It requires specific diagnostic skills and treatment. As such specialised tools are desirable as they can distinguish between non-neuropathic and neuropathic elements of pain. There is no consensus on which tool to use, but some of those most frequently employed include: Leeds assessment of neuropathic symptoms and signs (LANSS) pain scale, Douleur Neuropathique 4 questions (DN4), painDETECT and neuropathic pain score (NPS). The LANSS pain scale is based on analysis of sensory description and examination of sensory dysfunction (allodynia and a pinprick test), with a score of 12 or more out of 24 indicating a neuropathic cause for the pain. It can be used as a screening tool, but is also sensitive to treatment effect (16). The DN4 is a 10-item clinician-administered questionnaire consisting of sensory descriptors and physical signs, designed to distinguish between neuropathic and non-neuropathic pain. It was developed based on a patient study of those with definite somatic or neural lesions, and has a similar sensitivity and specificity to LANSS (17). The painDETECT scale was a questionnaire originally developed to distinguish between neuropathic and mechanical causes of lower back pain. It was validated in Germany, and found to have a high sensitivity, specificity and positive predictive value (18). It has been used to assess for neuropathic pain in a wide range of conditions including following total knee arthroscopy and in post-operative patients with head and neck cancer (19,20). The NPS measures neuropathic pain severity based on patient reported intensity of 11 descriptors, including how “sharp”, “hot”, “itchy” or “unpleasant” the pain is. It has been shown to have sensitivity to treatment effect (21).
Cancer pain
Pain is a one of many symptoms in patients with cancer, and can be assessed using the scales above. However, a more in-depth picture of the patients’ condition can be analysed using the memorial symptom assessment scale (MSAS) and its short form (MSAS-SF), which look at other symptoms and common disabilities in palliative care. It is validated for use in cancer patients, and is a multidimensional symptom assessment instrument that captures patient rated severity, frequency, and distress associated with 32 highly prevalent symptoms. It has been used in studies of patients with ovarian carcinoma, breast carcinoma, head and neck carcinoma, and cancer pain. The short form was developed to make patient self-reporting more straightforward and less time consuming to complete. The subscales of the MSAS-SF include the Global Distress Index, the Physical Symptom Distress Score and the Psychologic Symptom Distress Score. Patients are asked to determine the frequency with which they are experiencing any symptoms over a 7-day period. The MSAS-SF has been validated against other scales used to assess functional status (Karnofsky performance status) and quality of life [Functional Assessment of Cancer Therapy-General (FACT-G)] in those with cancer (22).
Elderly
The incidence of chronic pain is significant in the elderly population, particularly among care home residents. NRS or facial pain scales have been used effectively in these patients. However, those with dementia present a more complex challenge, as they may not have the ability to self-report. In these cases it may be necessary to observe pain behaviours or facial expression. An example is the mobilisation-observation-behaviour-intensity-dementia-2 (MOBID-2) pain scale, which is a staff-administered tool for dementia patients that has been found to be sensitive to a decrease in pain intensity after pain treatment over time (23). It is conducted in two parts. In Part 1, carers assess musculoskeletal pain by observing pain behaviour during five standardised movements of different body parts, and then rate the pain intensity. This may reveal pain previously masked by the patient avoiding movement. In Part 2, pain originating from internal organs, the head and the skin is recorded by carers based on pain behaviours monitored over time, localization of pain on pain drawings and inferred pain intensity (24).
Paediatric
Assessment of pain can be even more difficult in the paediatric population, particularly in neonates, pre-verbal children or those with severe handicap. In these situations and in children under the age of 3 where self-reporting is less reliable, the most commonly used alternative is an observational pain scale. The PedIMMPACT recommendations for pain assessment tools include the FLACC (face, legs, arms, cry, consolability) scale for procedural or post-operative pain, the COMFORT scale for those on critical care, and the PPPM (parents postoperative pain measure) for pain assessment at home. The FLACC scale is one of the most widely used, with extensive reliability and validity data available. The rater gives each of the 5 variables a score of 0–2, to give a total overall score of 0–10. The COMFORT Scale measures alertness, calmness/agitation, respiration, physical movement, blood pressure change, heart rate change, muscle tone, and facial tension. Extensive validity data are available for the COMFORT Scale (4).
Self-report scales can be used in slightly older children. The PedIMMPACT recommendations for these include the Poker-Chip Tool or “pieces of hurt” for those aged 3–4 years, the faces pain scale-revised (FPS-R) for those 4–12 years and VAS for those over 8 years old. The poker-chip tool asks children to say how many “pieces of hurt” they have right now, with one chip indicating “a little hurt” and 4 indicating “most hurt you could ever have”. At present VAS scores are used in preference to NRS, as there is insufficient data on NRS in the paediatric population. This is a potential area for future research (4).
Psychological factors
When assessing the effectiveness of analgesic treatment, it is important to consider compounding variables that may be affecting the patients pain experience. There is a well-documented relationship between pain and mood, which suggests that mood and emotions can influence pain conceptualisation, intensity and response to treatment. Instruments have been developed to assess depressed mood, mood disturbance and symptoms of emotional distress in chronic pain patients, such as the Beck depression inventory (BDI) and the profile of mood states (POMS), which have been recommended for use in chronic pain clinical trials (6). Catastrophizing, the tendency to ruminate and magnify pain, along with depression is a risk factor for a number of adverse long-term pain-related outcomes such as: physical disability; increased severity of pain; enhanced pain sensitivity and poor treatment outcomes, particularly in chronic rheumatic conditions (25). The Pain Catastrophizing scale describes different thoughts or feelings associated with pain, and a high score (greater than 30 out of 52) indicates significant catastrophizing, which is a risk factor for severe post-operative pain and development of chronicity (26). A study analysing the influence of preoperative psychological factors on postoperative pain and dysfunction following total knee arthroplasty, found that pain catastrophizing was a unique predictor of postoperative pain severity at 6 weeks. This suggests that interventions targeted to managing pain-related psychological risk factors might improve post-surgical pain (27). There has been extensive research into pain and biopsychosocial factors, however there needs to be clarification as to when to eliminate or attenuate negative emotions, and when to access, experience, and express them (28).
Summary
In order to determine how effective analgesia or a pain management plan is, it is essential to comprehensively assess pain intensity and its response to treatment. In the acute setting this can be done using clinical assessment and various pain rating scales including the VAS, NRS, VRS and FPS-R, with VAS and NRS having the greatest validity. Chronic pain can be more complex, and as such the tools used to assess it must reflect this. Options include in-depth multi-dimensional scales such as the MPQ and BPI, functional assessment such as the Pain Disability Index, and psychological analysis reviewing mood and coping ability. There is no consensus as to which tool is best for neuropathic pain, but options include the LANSS pain scale, DN4, painDETECT and NPS. Specific tools are available for use in more challenging population groups such as those with dementia (MOBID-2) and paediatric patients (FLACC, COMFORT, Poker-chip tool). See Table 2 for a summary of the assessment tools discussed in this article.
Table 2
| Pain assessment tool | Measurement | Summary of use |
|---|---|---|
| VAS | Acute pain, post-operative pain | 100 mm line: no pain to worst pain imaginable |
| NRS | Acute pain, post-operative pain, paediatric patients (over 4 years) | 0–10 (no pain to worst pain) |
| VRS | Acute pain, post-operative pain | No pain, mild, moderate, severe |
| Faces pain scale, e.g., FPS-R, Wong Baker FACES pain scale | Paediatric pain | Variations on line drawings of faces showing increasing levels of distress |
| MPQ-SF | Chronic pain | Sensory, affective and evaluative words which patients must rate and create PRI + present pain intensity rating |
| BPI | Chronic pain | Diagram to indicate site of pain + 11 point NRS + impact of pain on functions of daily living |
| Pain disability index | Chronic & pain related disability | 11 point NRS used to assess pain and disability across 7 different activities |
| Rowland-Morris questionnaire | Chronic lower back pain | 24 statements of physical function that patients are asked to indicate affect them |
| ODI | Chronic lower back pain | Patients rate their disability in 10 areas associated with lower back pain |
| MPS | Pelvic fracture outcomes (functional) | 5 subscales: pain; work; sitting; sexual intercourse and standing (sub-divided into walking aids, unaided gait, and walking distance). These are graded by the patient to give a score from 0 to 100 (graded: poor <55, fair 55–69, good 70–84, excellent ≥85), where higher scores represent the best outcomes |
| MSAS-SF | Cancer pain | Patient rated severity, frequency, and distress associated with 32 highly prevalent symptoms related to cancer; global distress index + physical symptom distress score + psychologic symptom distress score |
| MOBID-2 pain scale | Elderly patients with dementia | Part 1: carers observe pain behaviour during five movements of different body parts, and then rate the pain intensity |
| Part 2: pain originating from internal organs, the head and the skin is recorded by carers based on pain behaviours monitored over time, localization of pain on pain drawings and inferred pain intensity | ||
| FLACC scale | Paediatric, usually post procedure/operation | The 5 variables are given a score of 0–2, to give a total overall score of 0–10 |
| COMFORT | Paediatric, usually in intensive care | Measures alertness, calmness/agitation, respiration, physical movement, blood pressure change, heart rate change, muscle tone, and facial tension |
| Poker-chip tool | Paediatric | Children say how many “pieces of hurt” they have right now, with one chip indicating “a little hurt” and 4 indicating “most hurt you could ever have” |
VAS, visual analogue scale; NRS, numeric rating scale; VRS, verbal rating scale; FPS-R, faces pain scale-revised; MPQ-SF, McGill pain questionnaire & short form; PRI, pain rating index; BPI, brief pain inventory; ODI, Oswestry disability index; MPS, Majeed pelvic score; MSAS-SF, memorial symptom assessment scale and its short form; MOBID-2, mobilisation-observation-behaviour-intensity-dementia-2; FLACC, face, legs, arms, cry, consolability.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Digestive Medicine Research for the series “Current Issues in Analgesia for Major Surgery”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-93/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-93/coif). The series “Current Issues in Analgesia for Major Surgery” was commissioned by the editorial office without any funding or sponsorship. LK served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R. editors. New York: Guilford Press, 2011:15-34.
- Breivik EK, Björnsson GA, Skovlund E. A comparison of pain rating scales by sampling from clinical trial data. Clin J Pain 2000;16:22-8. [Crossref] [PubMed]
- Jensen MP, Chen C, Brugger AM. Postsurgical pain outcome assessment. Pain 2002;99:101-9. [Crossref] [PubMed]
- McGrath PJ, Walco GA, Turk DC, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain 2008;9:771-83. [Crossref] [PubMed]
- Hayes C, Browne S, Lantry G, et al. Neuropathic pain in the acute pain service: a prospective study. Acute Pain 2002;4:45-8. [Crossref]
- Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth 2013;111:19-25. [Crossref] [PubMed]
- Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975;1:277-99. [Crossref] [PubMed]
- Jumbo SU, MacDermid JC, Packham TL, et al. Reproducibility: reliability and agreement parameters of the revised short McGill pain questionnaire version-2 for use in patients with musculoskeletal shoulder pain. Health Qual Life Outcomes 2020;18:365. [Crossref] [PubMed]
- Jumbo SU, MacDermid JC, Kalu ME, et al. Measurement properties of the brief pain inventory-short form (BPI-SF) and revised short McGill pain questionnaire version-2 (SF-MPQ-2) in pain-related musculoskeletal conditions: a systematic review. Clin J Pain 2021;37:454-74. [Crossref] [PubMed]
- Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap 1994;23:129-38. [PubMed]
- Pollard CA. Preliminary validity study of the pain disability index. Percept Mot Skills 1984;59:974. [Crossref] [PubMed]
- Tait RC, Pollard CA, Margolis RB, et al. The pain disability index: psychometric and validity data. Arch Phys Med Rehabil 1987;68:438-41. [PubMed]
- Roland M, Fairbank J. The Roland-Morris disability questionnaire and the Oswestry disability questionnaire. Spine (Phila Pa 1976) 2000;25:3115-24. [Crossref] [PubMed]
- Majeed SA. Grading the outcome of pelvic fractures. J Bone Joint Surg Br 1989;71:304-6. [Crossref] [PubMed]
- Kleweno C, Vallier H, Agel J. Inaccuracies in the use of the Majeed pelvic outcome score: a systematic literature review. J Orthop Trauma 2020;34:63-9. [Crossref] [PubMed]
- Bennett M. The LANSS pain scale: the Leeds assessment of neuropathic symptoms and signs. Pain 2001;92:147-57. [Crossref] [PubMed]
- Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005;114:29-36. [Crossref] [PubMed]
- Freynhagen R, Baron R, Gockel U, et al. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911-20. [Crossref] [PubMed]
- Hasegawa M, Tone S, Naito Y, et al. Possible neuropathic pain in patients with osteoarthritis of the knee before and after total knee arthroplasty. J Pain Res 2021;14:3011-5. [PubMed]
- Gostian M, Loeser J, Albert C, et al. Postoperative pain treatment with continuous local anesthetic wound infusion in patients with head and neck cancer: a nonrandomized clinical trial. JAMA Otolaryngol Head Neck Surg 2021;147:553-60. [Crossref] [PubMed]
- Jensen MP, Dworkin RH, Gammaitoni AR, et al. Assessment of pain quality in chronic neuropathic and nociceptive pain clinical trials with the Neuropathic Pain Scale. J Pain 2005;6:98-106. [Crossref] [PubMed]
- Chang VT, Hwang SS, Feuerman M, et al. The memorial symptom assessment scale short form (MSAS-SF). Cancer 2000;89:1162-71. [Crossref] [PubMed]
- Husebo BS, Ostelo R, Strand LI. The MOBID-2 pain scale: reliability and responsiveness to pain in patients with dementia. Eur J Pain 2014;18:1419-30. [Crossref] [PubMed]
- Husebo BS, Strand LI, Moe-Nilssen R, et al. Pain in older persons with severe dementia. Psychometric properties of the Mobilization-Observation-Behaviour-Intensity-Dementia (MOBID-2) Pain Scale in a clinical setting. Scand J Caring Sci 2010;24:380-91. [Crossref] [PubMed]
- Edwards RR, Cahalan C, Mensing G, et al. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol 2011;7:216-24. [Crossref] [PubMed]
- Sullivan MJL, Bishop SR, Pivik J. The Pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524-32. [Crossref]
- Sullivan M, Tanzer M, Stanish W, et al. Psychological determinants of problematic outcomes following Total Knee Arthroplasty. Pain 2009;143:123-9. [Crossref] [PubMed]
- Lumley MA, Cohen JL, Borszcz GS, et al. Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol 2011;67:942-68. [Crossref] [PubMed]
Cite this article as: Parrott N, Kelliher L. How do we assess analgesia efficacy?—a narrative review. Dig Med Res 2022;5:12.


