A rare incidence of recurrent Boerhaave’s syndrome: an alternative operative approach—case report
Introduction
Hermann Boerhaave, a Dutch professor of medicine, first described spontaneous transmural rupture of the oesophagus as a result of high intra-oesophageal pressures related to emesis in 1724 (1). Recurrent episodes are an even rarer phenomenon. There is no consensus on the optimal approach to treatment. We report a case of recurrent Boerhaave’s syndrome and the use of an abdominal approach in operative management, not described previously. We also undertook a summative review and analysed the common risk factors that may alert clinicians of this condition and hence a lower threshold for imaging, to expedite surgical intervention as early repair is imperative and associated with better outcome. We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/dmr-21-72).
Case presentation
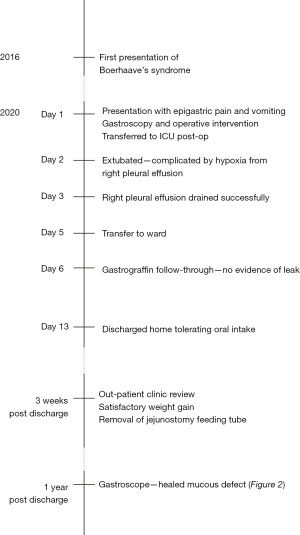
A 51-year-old man presented with a recurrent episode of Boerhaave’s syndrome. His first episode presented with sudden onset epigastric pain on the background of nausea and vomiting several days earlier. He required an emergency right thoracotomy and primary oesophageal repair, with an uncomplicated post-operative stay in the intensive care unit for 3 days and was discharged on day 15. He was commenced on pantoprazole indefinitely. Further follow up confirmed Barrett’s oesophagus. He continued to experience ongoing reflux symptoms and reduced appetite with a repeat gastroscope confirming a long segment of Barrett’s oesophagus.
This patient then re-presented emergently four years after his initial episode with multiple vomits and nausea followed by acute epigastric and right upper quadrant pain. On clinical examination, he was found to have epigastric and right upper quadrant tenderness and guarding, tachycardia, tachypnoea, normotension and temperature of 36.1 °C. Blood tests included a troponin, lipase and liver function tests which were normal, and a mildly elevated C-reactive protein. Electrocardiogram (ECG) showed sinus tachycardia with no ischaemic changes. Chest X-ray had no free gas seen under the diaphragm and was essentially unremarkable.
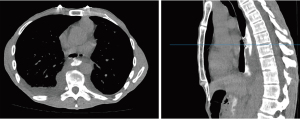
An urgent computed tomography (CT) abdomen with oral contrast was performed, revealing a focal defect in the anterior wall of the distal oesophagus associated with extravasation of contrast into the lower mediastinum and bilateral pleural effusions (Figure 1). A distal oesophageal perforation was diagnosed.
Given the distal location of the perforation and confinement of contamination to the mediastinal space, an abdominal approach to the recurrent Boerhaave’s syndrome was used via a midline laparotomy. The anterior distal oesophageal perforation was visualised 4cm from the hiatus and repaired primarily, along with a supporting omental patch. The previous perforation site was located from old sutures, just superior to the site of his recurrent perforation. On-table endoscopy was then performed with no air leak found. The right chest cavity was also debrided through the hiatus.
A feeding jejunostomy was fashioned and nasogastric tube inserted with subsequent transfer to the intensive care unit. Post-operative gastrografin follow-through at day 6 showed no evidence of leak. His recovery was complicated by a large right pleural effusion which was successfully drained percutaneously. He was subsequently discharged on day 13 tolerating oral intake.
The patient was reviewed 3 weeks later and reported a smooth recovery with weight gain. The feeding jejunostomy was removed at that time. Follow-up gastroscopy showed a healed mucosal defect (Figure 2).
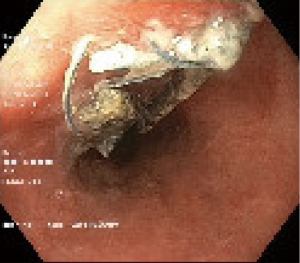
There was no evidence of underlying oesophagitis. In summary, this patient had recurrent spontaneous oesophageal perforation, or recurrent Boerhaave’s syndrome. The management was summarised on a timeline in Figure 3.
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Spontaneous oesophageal perforation was first described in 1724 by Herman Boerhaave, a Dutch physician that bequeathed the name. It is a primary oesophageal perforation in the context of high intra-oesophageal pressures. It is itself a rare condition and is associated with the highest mortality of any gastrointestinal perforation (2). Reperforation is even rarer with only 11 cases found in recent literature reviews (3-5). Although Boerhaave’s syndrome typically presents with a triad of vomiting, chest pain and subcutaneous emphysema [the Mackler triad (1)], our patient did not have the classical syndrome on both occasions. Given the lack of precedent cases of recurrence, identifying patients at risk of reperforation is difficult. There is no consensus on the optimal approach to management.
Of the eleven cases of recurrent Boerhaave’s, five were managed operatively, two endoscopically and four conservatively. While the majority of cases opted for surgery, the high rate of non-surgical management may be a manifestation of prior inflammation and adhesions, which can result in a contained perforation preventing widespread contamination throughout the mediastinum and pleura that would then require emergent surgical intervention.
However, our case is the first to report recurrent Boerhaave’s syndrome managed surgically by abdominal approach at the second episode following previous thoracotomy. While typically managed with a thoracotomy in this location, prior reports mention dense adhesions and chronic pleural inflammation encountered in repeat thoracotomy in recurrent Boerhaave’s syndrome (6,7). To this end an abdominal approach was used, knowing that trans-hiatal dissection would allow access to a distal perforation and pleural contamination, while avoiding adhesions.
Classically, if a surgical approach is indicated, the decision to treat with a thoracotomy or abdominally depends on the location of the defect. Tears of the mid-oesophagus undergo a right thoracotomy while in the distal segment, a left thoracotomy is often used (8). Abdominal approach is used for tears of abdominal oesophagus with peritoneal soiling (9).
We have found the trans-abdominal approach to managing Boerhaave’s syndrome particularly advantageous in similar scenarios. Following this principle, the trans-abdominal approach can also be used if previous thoracic surgery has been performed on patients presenting with primary Boerhaave’s syndrome when the perforation on imaging is within 5 cm of the hiatus, with no evidence of pleural contamination. By avoiding a thoracotomy, we prevent use of single lung ventilation and the associated complications and resulting physiological insult (9) as well as avoiding likely adhesions in the thorax.
In patients unsuitable for thoracotomy or too unstable for invasive surgical management, an endoscopic approach to recurrent Boerhaave’s syndrome has been described with use of over-the-scope clips (4) and oesophageal stenting (5). A range of techniques have been successfully employed and can be considered versus opting for conventional thoracotomy often used in initial presentations. To our knowledge, there are no guidelines available for recurrent Boerhaave’s syndrome, likely given the rarity of its presentation.
We have attempted to identify risk factors associated with patients reported with recurrent Boerhaave’s syndrome. Table 1 presents risk factors and symptoms of second presentations described in previous case reports. Of note, the most common described risk factor appears to be alcohol intake, of amounts high enough to result in withdrawal symptoms and delirium tremens as a cause for vomiting (7).
Table 1
| Author | Age | Interval between recurrence | Alcohol use | Other risk factors | Symptoms of recurrent perforation |
|---|---|---|---|---|---|
| Saha SP (1979) | 17 | 6 months | N/A | N/A | Lower anterior thoracic pain with mild dysphagia |
| Nakata Y (2001) | 74 | 5 days | N/A | N/A | Chest pain after vomit |
| Ieta K (2013) | 43 | 6 years | Mod-heavy | BMI 17.4 | Severe epigastric pain with slightly bloody vomitus after drinking alcohol |
| Naitoh H (2014) | 52 | 8 years | N/A | Anti-phospholipid syndrome | Haematemesis following severe vomits |
| Khan OA (2005) | 59 | 26 months | N/A | Barrett’s oesophagus | Right sided chest pain and haematemesis |
| Khan OA (2005) | 49 | 27 months | N/A | Barrett’s oesophagus | Severe right sided chest pain |
| Reeder LB (1995) | 66 | 30 years | Mod-heavy | Peptic ulcer disease | Sudden sharp epigastric pain radiating to left shoulder followed by several episodes of haematemesis |
| Wang SC (2016) | 54 | 18 months | Heavy | N/A | Dyspnoea and vomiting |
| Bakarat M (2018) | 62 | 12 years after 1st, 4 years after 2nd | Heavy | Barrett’s oesophagus | Dyspnoea, severe persistent chest and epigastric pain radiating to shoulder and neck |
| Lujan HJ (1997) | 45 | 8 months | Heavy | Cocaine abuse | Sudden sharp right sided chest pain |
| Kish GF (1980) | 51 | 14 months | N/A | N/A | Chest pain and respiratory insufficiency |
However, while our patient did not drink alcohol, his low body mass index (BMI) of 17 may have predisposed him to recurrent perforation. Another patient with recurrent perforation was also reported to have a similar BMI of 17.4 (7). Our patient also had Barrett’s oesophagus similar to three other cases of reperforation (4,10). It is known that spontaneous perforation can be a result of inflammation and oesophageal dysmotility (11), combined with a weak mucosa and raised intrabdominal pressures. Our patient’s low BMI may have contributed to oesophageal wall weakness predisposing him to recurrence, in the setting of his multiple vomits prior to presentation.
This table shows that patient characteristics and symptoms are fairly non-specific. The age of patients ranges from 17 to 74 years old with the time frame between reperforations ranging from 5 to 30 years. Furthermore, symptoms in the recurrent episode are non-specific, leading to a delayed diagnosis of Boeerhave’s syndrome—as often occurs with primary Boerhaave’s syndrome (12).
Given that time to surgery within 24 hours is a known factor for significantly improved outcomes (2), a lower threshold for urgent imaging in patients with a past history of Boerhaave’s syndrome that present with even mild symptoms may help to expedite treatment.
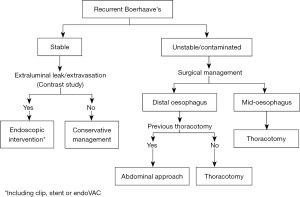
This case describes a rare occurrence of recurrent Boerhaave’s syndrome four years after initial presentation. Ours is the first to describe the use of an abdominal approach in this setting with a successful outcome, offering a viable alternative with the advantage of avoiding scarring from previous thoracotomy. Based on our experience of this case, and the reported cases in the literature, we propose an algorithm for the management of recurrent Boeerhave’s syndrome shown in Figure 4. From our analysis of the reported cases in the literature, we suggest a lower threshold for suspecting a recurrence in Boerhaave’s syndrome given the high mortality of the condition, and the non-specific nature of patient characteristics and presenting symptoms.
Acknowledgments
We thank Mr Ming Kon Yii for his contributions in editing and reviewing this case report.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/dmr-21-72
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/dmr-21-72). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Turner AR, Turner SD. Boerhaave Syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021.
- de Schipper JP, Pull ter Gunne AF, Oostvogel HJM, et al. Spontaneous Rupture of the Oesophagus: Boerhaave’s Syndrome in 2008. Dig Surg 2009;26:1-6. [Crossref] [PubMed]
- Rokicki M, Rokicki W, Moj M, et al. Boerhaave Syndrome - over 290 years of surgical experiences. Can the disorder recur? Pol Przegl Chir 2018;91:27-9. [Crossref] [PubMed]
- Barakat MT, Girotra M, Banerjee S. (Re)building the Wall: Recurrent Boerhaave Syndrome Managed by Over-the-Scope Clip and Covered Metallic Stent Placement. Dig Dis Sci 2018;63:1139-42. [Crossref] [PubMed]
- Wang SC, Scott WW Jr. Recurrent Spontaneous Esophageal Rupture Managed With Esophageal Stenting. Ann Thorac Surg 2016;102:e5-6. [Crossref] [PubMed]
- Lujan HJ, Lin PH, Boghossian SP, et al. Recurrent spontaneous rupture of the esophagus: an unusual late complication of Boerhaave's syndrome. Surgery 1997;122:634-6. [Crossref] [PubMed]
- Ieta K, Oki A, Teshigahara K, et al. Recurrent spontaneous esophageal rupture. Clin J Gastroenterol 2013;6:33-7. [Crossref] [PubMed]
- Mavroudis CD, Kucharczuk JC. Acute Management of Esophageal Perforation. Curr Surg Rep 2014;2:34. [Crossref]
- Khan AZ, Forshaw MJ, Davies AR, et al. Transabdominal approach for management of Boerhaave's syndrome. Am Surg 2007;73:511-3. [Crossref] [PubMed]
- Khan OA, Barlow CW, Weeden DF, et al. Recurrent spontaneous esophageal rupture. Eur J Cardiothorac Surg 2005;28:178-9. [Crossref] [PubMed]
- Salo JA, Seppälä KM, Pitkäranta PP, et al. Spontaneous rupture and functional state of the esophagus. Surgery 1992;112:897-900. [PubMed]
- Tonolini M, Bianco R. Spontaneous esophageal perforation (Boerhaave syndrome): Diagnosis with CT-esophagography. J Emerg Trauma Shock 2013;6:58-60. [Crossref] [PubMed]
Cite this article as: Yii E, Wong E, Joglekar S, Johnson MA. A rare incidence of recurrent Boerhaave’s syndrome: an alternative operative approach—case report. Dig Med Res 2021;4:76.