The role of endoscopic ultrasound and magnetic resonance cholangiopancreatography in the diagnosis of idiopathic recurrent acute pancreatitis: a narrative review
Introduction
Acute pancreatitis (AP) is the most common gastrointestinal condition requiring hospitalization in the United States, amounting to a cost of $2.6 billion in 2009 (1). Gallstones and alcohol intake underly the etiology in approximately 70–80% of episodes of AP. About 10–30% of cases have no clear cause. Idiopathic acute pancreatitis (IAP) is diagnosed when initial laboratory testing (including a lipid panel and calcium level) and non-invasive imaging [transabdominal ultrasound (TUS) and/or computer tomography (CT) scan] fail to identify an etiology for the first episode of pancreatitis (2). About 25–70% of patients with IAP develop recurrent episodes within 3 years, and become classified as having idiopathic recurrent acute pancreatitis (IRAP) (3,4). Recurrent acute pancreatitis is defined as at least two AP episodes with resolution of symptoms between each episode, in the absence of chronic pancreatitis (2).
The true prevalence of IAP remains unknown as the extent of diagnostic evaluation before considering the diagnosis is not well established and ambiguity exists around the best diagnostic approach of finding an underlying etiology for IAP/IRAP. The most recent guidelines of the International Association of Pancreatology (IAP) and the American Pancreatic Association (APA) differ from those of the American College of Gastroenterology (ACG) on the diagnostic approach for patients with IAP/IRAP (2,5). Whereas the IAP/APA guidelines recommend endoscopic ultrasound (EUS) after an initial episode of IAP and further recommend a secretin-stimulated magnetic resonance imaging cholangiopancreatography (S-MRCP), the ACG guidelines advise for limited endoscopic investigation (2,5). The extent of investigation to unearth a cause for IAP/IRAP is an important clinical dilemma.
Both guidelines were published in 2013. New data have been published since then. The key question is: what should be the diagnostic approach to patients with ‘presumed’ IAP/IRAP eight years after the guidelines? Given the uncertainty around the extent of diagnostic evaluation, our aim for this narrative review is to systematically assess the literature to formulate an evidence-based diagnostic approach to patients with IAP/IRAP. We also briefly discuss the terminology and etiologies when considering IAP/IRAP, and then discuss the evidence behind the different modalities of evaluation.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/dmr-21-66).
Methods
Searches were conducted in sequential order using the databases of PubMed/MEDLINE, Embase and then Cochrane Library on recurrent idiopathic pancreatitis as outlined in (Table 1). The search date was set to include articles published between January 1st, 2000, and August 1st, 2021. Only studies published in English were included. Studies focused on pediatric patients were excluded as this narrative review was intended to focus on adult patients. Randomized trials, prospective and retrospective cohort studies, expert editorials, systematic reviews, and meta-analyses were included, whereas case reports and review articles were excluded. In rare cases, articles were excluded if both their full-text and abstract were unavailable. Irrelevant studies, defined as studies not pertaining to idiopathic pancreatitis after review of the article’s title and abstract, were excluded.
Table 1
| Database | Years searched | Searched terms | Number of items |
|---|---|---|---|
| PubMed/MEDLINE | January 1, 2000–August 1, 2021 | ((idiopathic [Title]) AND (pancreatitis [Title])) AND (recurrent) | 104 |
| Embase | January 1, 2000–August 1, 2021 | “recurrent” (all fields) AND “idiopathic” (title) AND “pancreatitis” (title) NOT ‘case report’ (quick search) |
175 |
| Cochrane Library | January 1, 2000–August 1, 2021 | “recurrent idiopathic pancreatitis” | 21 |
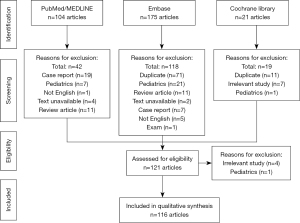
During the first phase of review, one assessor searched the three databases to collect articles on recurrent idiopathic pancreatitis based on their titles and abstracts. The search yielded 104 items from PubMed/MEDLINE, 175 items from Embase, and 21 from Cochrane Library. Manual assessment of the title and abstract of the articles led to the exclusion of 179 out of 300 articles, mostly for being duplicates (Figure 1). After the first round of review, the articles were then assessed for eligibility by two assessors based on their full-text, and 5 additional articles were further excluded. Discrepancy between the two assessors was arbitrated by a third assessor. A total of 116 articles were reviewed in the qualitative synthesis to formulate a diagnostic approach to determine the etiology of IAP. Given this narrative review focused on the diagnostic approach, excluding genetic testing, and not the management, or on endoscopic retrograde cholangiopancreatography (ERCP), a further 38 articles were not included in the discussion of the manuscript.
Discussion
Definition of ‘presumed’ IAP
The definition of IAP varies across studies and guidelines, which makes it difficult to determine its ‘true’ prevalence with one study estimating it to be 20% (6). Studies that have used stricter criteria to define IAP have found a lower prevalence of 12% (7). Therefore, a new term, ‘presumed’ IAP, which takes into consideration of what should constitute a sufficient and adequate work up before considering IAP, has been coined (7-9). ‘Presumed’ IAP should be considered after a standard evaluation that includes: (I) a detailed patient history (alcohol use, recent ERCP, medications associated with pancreatitis, recent abdominal trauma or abdominal surgery, hereditary or familial pancreatitis, and a family history of cystic fibrosis); (II) laboratory testing (triglycerides, corrected calcium, alanine aminotransferase, and immunoglobulin G, subclass 4 (IgG4) levels at admission); (III) readily available imaging (TUS at admission, and repeat TUS at 8–12 weeks following the initial episode of AP) (7-9). For reference, medications that have been associated with AP are listed in (10,11). If the etiology is not clear after this standard workup, then a diagnosis of ‘presumed’ IAP should be made.
What etiologies should be considered?
Determining the etiology of AP is essential as it determines the management, both in the acute phase and to prevent recurrence. The most common causes of AP include gallstones and alcohol. In mild gallstone pancreatitis without necrosis, a same-admission laparoscopic cholecystectomy is recommended to prevent recurrences (2,12). Less common causes of pancreatitis include autoimmune, drug-induced, hypertriglyceridemia, hypercalcemia, trauma, recent ERCP or abdominal surgery, familial or hereditary causes, and pancreaticobiliary neoplasms. When the initial standard workup is inconclusive, then an occult etiology for the ‘presumed’ IAP should be sought. Occult etiologies of IAP include: (I) presence of biliary stones, microlithiasis or sludge previously undetected; (II) chronic pancreatitis; (III) pancreaticobiliary neoplasms including intraductal papillary mucinous neoplasm (IPMN); (IV) presence of anatomic abnormalities [annular pancreas, pancreatic divisum (PD), anomalous pancreaticobiliary junction (APBJ), or choledochocele]; (V) sphincter of Oddi dysfunction (SOD), and (VI) hereditary mutations. A less common etiology of AP is steatopancreatitis, also known as non-alcoholic fatty pancreas disease (NAFPD), which has not been extensively researched (13). Only one study has evaluated steatopancreatitis in patients with IRAP, and found intraparenchymal pancreatic fat is increased in patients with IRAP compared to matched controls (14).
There is disagreement in the field on the certainty of PD, IPMN, and SOD as causes of IRAP. In a cross-sectional study of 46 patients with IAP/IRAP compared with 500 healthy controls, the prevalence and rate of PD was significantly higher for patients with IRAP, but not for patients with IAP. Furthermore, multiple logistic regression showed that the presence of PD increased the odds of pancreatitis by 23.4 times compared to healthy controls (15). Additional studies have found associations between genetic mutations (SPINK, PRSS1, and CFTR mutations, and L26V and r12338 polymorphisms in cathepsin B gene) and IRAP in patients with PD compared to those without PD, indicating PD may be a risk factor for the development of IRAP in the presence of an underlying genetic predisposition (16-18). The association between IPMN and pancreatitis has been less well studied. In the largest study on IPMN-associated pancreatitis, only 7% of 489 patients with IPMN developed pancreatitis, and the rate was significantly higher in those with main pancreatic duct (MPD) IPMN, than those with branch-duct (BD)-IPMN, 14% vs. 5% (19). SOD as an etiology of IAP is a controversial topic as it is unclear if SOD is a precursor or a complication from recurrent acute pancreatitis. Interestingly, however, the prevalence of SOD in patients with IAP has been reported to be between 30–65% (20). Further research is required in this field.
Occult causes may be evaluated by three modalities: advanced imaging [magnetic resonance cholangiopancreatography (MRCP)], endoscopic ultrasound (EUS), and rarely ERCP. It is estimated that an etiology is discoverable in more than 90% of cases after “extensive” evaluation, including EUS and ERCP (21). The best diagnostic approach, however, remains controversial.
Repeated TUS or EUS?
The most common etiology discovered as an occult cause of IAP/IRAP is biliary disease (gallstones, biliary sludge, or microlithiasis), being estimated to account for 30–80% of cases (i.e., untreated gallstone pancreatitis) (8). Additionally, the presence of these occult gallstones increases with age in patients with IRAP (22). Although TUS has a moderate sensitivity for detecting gallstones, reported as 84% (23), it is inferior to EUS, which has a sensitivity of 100% in patients with AP (24). Additionally, TUS may have a lower sensitivity for biliary disease during AP, therefore, the IAP/APA guidelines recommend obtaining a repeat TUS after the inflammation has subsided (5). A post-hoc analysis of a prospective cohort study of 176 patients with ‘presumed’ IAP found a repeat TUS may detect biliary disease in 21% of cases (7). In a prospective study of 24 patients, serial TUS at 3 month intervals was performed for patients with ‘presumed’ IAP who had undergone MRCP that was negative for chronic pancreatitis, tumors, PD, or biliary sludge (25). The study found 16 out of 24 (67%) of patients were found to have biliary disease at a median follow-up period of 33 months, of which 14 underwent cholecystectomy with only 1 patient having recurrent pancreatitis (25). Interestingly, a meta-analysis on 9 studies of 526 patients with ‘presumed’ IAP found that those who underwent cholecystectomy (n=126) had significantly reduced rates of recurrent attacks than those who followed conservative management (11.1% vs. 35.2%, risk ratio 0.44, 95% CI: 0.27–0.71) (26). Even after further workup with EUS and MRCP, the rates of recurrent attacks remained lower in the cholecystectomy group (11.0% vs. 38.9%, risk ratio 0.41, 95% CI: 0.16 to 1.07).
Despite the utility of repeating a TUS, the role of serial TUS for IRAP remains unclear as studies on the diagnostic yield of serial TUS are limited. Importantly, however, serial TUS may prove to be limited in obese patients given the decreased sensitivity of TUS for the detection of occult biliary disease in this patient population. Another area of uncertainty is whether serial TUS is more cost-effective at diagnosing a cause of IAP/IRAP compared to EUS after an initial repeat TUS. Based on the available data, however, an EUS should be the next step after a negative repeat TUS in all patients. Further studies are needed to determine which approach has the highest diagnostic yield.
Numerous studies have been performed to determine the diagnostic utility of EUS, showing a yield between 32% to 88% for detecting an etiology for IAP/IRAP (4,8,25,27-47). A 2020 meta-analysis included 22 studies, such as retrospective, prospective, and post-hoc analyses, to examine the yield of EUS in patients with IAP/IRAP (9). Notably, the analysis noted none of the individual studies performed a complete standard diagnostic work-up according to the IAP/APA guidelines. Only two studies (31,36) had performed a repeat TUS after the initial episode of AP. Out of 1490 patients with IAP, EUS detected an etiology in 59% of patients (95% CI: 52–66%), including biliary disease (30%), chronic pancreatitis (12%), and neoplasms (2%). The total number of neoplasms was 43, and included 22 benign IPMNs, 12 pancreatic cancers, and one malignant IPMN. Notably, the prevalence of cancer as a cause of AP has been associated with increasing age, especially between 56 to 70 years (48).
However, the diagnostic yield depends on multiple factors, including the patient population and the workup prior to EUS. As the definition of ‘presumed’ IAP is not standardized, studies have included a heterogenous population of patients believed to have IAP. In the meta-analysis, a quality assessment found that 21 out of the 22 studies selected patients that were not strictly representative of patients with ‘presumed’ IAP, which indicates a general criticism of the field of research on idiopathic pancreatitis. Given only a minority of these studies included a repeat TUS, the diagnostic yield of EUS is most likely overestimated.
Nonetheless, EUS plays a critical role in the assessment of patients with ‘presumed’ IAP/IRAP as it provides insight into the management approach, risk factors for recurrence, and progression to chronic pancreatitis. First, studies have shown that EUS is as useful after a single attack of pancreatitis as compared to after recurrent attacks (4,7,46,49). A post-hoc analysis of 176 patients with IAP who underwent additional testing, 36 patients with a single episode of IAP and 26 with IRAP underwent EUS with diagnostic yields of 36% and 35%, respectively (7). Similarly, a retrospective study found no statistically significant difference in the diagnostic yield of EUS in patients with IAP compared to IRAP (65.4% vs. 71.4%, respectively) (49). The other two studies showed similar overall diagnostic yields after a single or recurrent attack of IAP including in patients with and without a prior cholecystectomy (4,46). Second, studies have shown that EUS may provide predictive factors on recurrent episodes. In a 10-year longitudinal prospective study of 201 patients with IAP/IRAP, a negative EUS was predictive of a lower prevalence of relapse in patients with a single attack (50). At a median follow-up of 37 months, the recurrence of pancreatitis for patients with IAP was 24% (95% CI: 15–38%) compared to 49% (95% CI: 38–62%) for patients with IRAP (50). Similarly, a retrospective study of 106 patients with IAP/IRAP found patients with IRAP referred for EUS had a higher rate of recurrence than those with IAP, 57.1% vs. 16.7%, P<0.001, at a mean follow-up of 53.6 months (49). Importantly, this study found three statistically significant predictors for recurrence: age <65 years (OR: 3.56), absence of biliary disease on EUS (OR: 2.87), and history of a prior cholecystectomy (OR: 3.19) (49). Third, studies have shown that EUS may predict which patients may progress to chronic pancreatitis. The detection of chronic pancreatitis has been shown to be highest in patients with IRAP than those with IAP despite gallbladder status (no cholecystectomy, 42.0% vs. 21.6%, P=0.0008; post-cholecystectomy, 38.6% vs. 16.4%, P=0.008) (46). Additionally, a large multicenter study of 669 patients (15% idiopathic) found that 25% of patients with IAP progressed to IRAP, and 10% to chronic pancreatitis. The study further found that having IAP was an independent risk factor for progression to recurrent pancreatitis (aOR 2.51, P=0.001) and chronic pancreatitis (OR 3.12, P=0.005) (51). This is supported by another study that 45.4% of IRAP patients progress to chronic pancreatitis at a mean follow-up of 43.5 months (52). Not surprisingly, biliary disease is more common in IAP compared to IRAP on EUS (46,49).
Taken together, these studies on EUS suggest three key points, which may help formulate an evidence-based diagnostic approach for patients with ‘presumed’ IAP.
- Without a clear definition of ‘presumed’ IAP, the diagnostic yield of EUS may be overestimated in current studies as the most frequent etiology of biliary disease may be detectable by less costly means, such as a repeat TUS.
- The diagnostic utility of EUS remains high and is similar regardless of if it is performed after a first or repeated attack of acute pancreatitis.
- EUS has a role in prognostication of recurrent attacks and progression to chronic pancreatitis, which may play role in management.
The Pancreatitis of Idiopathic origin: Clinical added value of endoscopic UltraSonography (PICUS) study, a recently established multicenter prospective cohort study, will enroll patients with an initial episode of IAP, who have undergone a standard diagnostic workup, including standardized history, laboratory testing, and conventional imaging with a repeat TUS (9). This study will exclude patients with recurrent pancreatitis (i.e., IRAP), and those with chronic pancreatitis. It will provide a clearer picture on the true prevalence of IAP/IRAP and diagnostic yield of EUS.
MRCP vs. S-MRCP or EUS?
An S-MRCP is a non-invasive imaging study to detect pathology in the pancreatic duct that is complementary to EUS. Secretin is a hormone produced by S cells lining the duodenal mucosa, which causes secretion of bicarbonate-rich fluid from the pancreatic ducts, temporarily leading to their dilatation. The main role of S-MRCP is to detect the presence of anatomical abnormalities, chronic pancreatitis, pancreaticobiliary neoplasms, and abnormal SOD function (53,54).
A meta-analysis of 34 studies with a total of 2,338 patients evaluated the diagnostic yield of EUS when compared to MRCP alone, or S-MRCP in patients with ‘presumed’ IAP (55). In 7 of the studies comparing EUS to MRCP, the diagnostic yield of EUS was higher than MRCP, 64% vs. 34% (P<0.001). Overall, the diagnostic yield was 60% (1,324 out of 2,200 patients), 24% (48 out of 195 patients), and 43% (62 out of 140 patients) in the EUS, MRCP, and S-MRCP groups, respectively. On subgroup analysis, EUS was superior to MRCP at detecting biliary disease (33% vs. 7%, respectively; P<0.001), but MRCP was non-inferior to EUS at detecting chronic pancreatitis (5% vs. 8%, respectively; P=0.37). However, S-MRCP was superior to EUS and MRCP alone at detecting anatomic abnormalities, such as PD (12% vs. 2% and 2%, respectively). Additionally, a retrospective study of 40 patients found that 50% of patients with IAP were found to have biliary disease on EUS after having a negative TUS, CT scan, and MRCP (39).
Taken together, these studies suggest that S-MRCP is superior to MRCP alone at detecting anatomic abnormalities, and non-inferior for chronic pancreatitis but inferior for biliary disease when compared to EUS. Some limitations of both S-MRCP and MRCP should be considered, however, and include limited availability at smaller health centers, higher costs, and unstable medical supply of secretin to perform the study. Given the limitations of MRCP, it should be performed only if it is readily available.
Summary of diagnostic approach
First, a clear definition of ‘presumed’ IAP should be established, both for clinical and research purposes. This definition should follow the current guidelines established by the IAP/APA, which recommend a serial TUS following an initial episode of AP to exclude biliary disease. Following this standard evaluation, a patient may be labeled as having ‘presumed’ IAP. An MRCP/S-MRCP should be performed only if readily available, and only after a repeat TUS is negative as the MRCP/S-MRCP may identify pancreaticobiliary neoplasms, anatomic abnormalities, and SOD. EUS should be considered superior to MRCP/S-MRCP due to its higher diagnostic yield.
Second, serial imaging with TUS or empiric cholecystectomy may be alternatives to pursuing an EUS after ‘presumed’ IAP/IRAP is established if EUS is not accessible. Risk and benefit discussion, with consideration of potential costs, should be held with the patient to determine the next best step.
Third, if EUS fails to identify a positive etiology, defined as (I) occult biliary disease, (II) chronic pancreatitis, or (III) neoplasms, then genetic testing for pathogenic gene variants associated with pancreatitis (56-79) (especially if the patient is <35 years of age) (64,71,79) should be considered, which is out of the discussion of this narrative review.
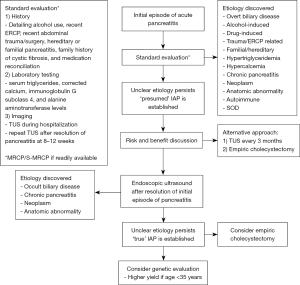
After the above extensive evaluation, an underlying etiology may be discoverable in >90% of patients as previously estimated. A graphical summary of the suggested diagnostic approach is provided in (Figure 2). With its discovery, the underlying cause for the IAP/IRAP should guide the subsequent management approach.
Conclusions
IAP poses a difficult clinical dilemma regarding its etiology and the most efficient approach to arriving to an occult cause. Regardless, most patients are found to have an underlying cause after extensive evaluation with occult biliary disease being the most common finding, followed by chronic pancreatitis, neoplasms, and anatomic abnormalities. Less likely etiologies include SOD and unknown genetic predispositions. With a step-by-step approach consisting of a standard evaluation, followed by EUS, an occult cause may be identified in patients with idiopathic pancreatitis. Further studies, however, are required to determine the most cost-effective diagnostic approach.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/dmr-21-66
Peer Review File: Available at https://dx.doi.org/10.21037/dmr-21-66
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/dmr-21-66). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fagenholz PJ, Fernández-del Castillo C, Harris NS, et al. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas 2007;35:302-7. [Crossref] [PubMed]
- Tenner S, Baillie J, DeWitt J, et al. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013;108:1400-15; 1416.
- Umans DS, Rangkuti CK, Sperna Weiland CJ, et al. Endoscopic ultrasonography can detect a cause in the majority of patients with idiopathic acute pancreatitis: a systematic review and meta-analysis. Endoscopy 2020;52:955-64. [Crossref] [PubMed]
- Govil A, Agrawal MK, Agrawal D, et al. Role of endoscopic ultrasonography in patients with first episode of idiopathic acute pancreatitis. Indian J Gastroenterol 2014;33:241-8. [Crossref] [PubMed]
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013;13:e1-15. [Crossref] [PubMed]
- Studd CR, Ayonrinde OT, Galhenage S. Characteristics of patients with idiopathic acute pancreatitis at a tertiary referral hospital. J Gastroenterol Hepatol 2009;24:A223.
- Hallensleben ND, Umans DS, Bouwense SAW, et al. The diagnostic work-up and outcomes of ‘presumed’ idiopathic acute pancreatitis: A post-hoc analysis of a multicentre observational cohort. United European Gastroenterol J 2020;8:340-50. [Crossref] [PubMed]
- Mohan BP. Diagnosis of idiopathic acute pancreatitis: the simpler, the better? Endoscopy 2020;52:965-6. [Crossref] [PubMed]
- Umans DS, Timmerhuis HC, Hallensleben ND, et al. Role of endoscopic ultrasonography in the diagnostic work-up of idiopathic acute pancreatitis (PICUS): study protocol for a nationwide prospective cohort study. BMJ Open 2020;10:e035504. [Crossref] [PubMed]
- Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet 2015;386:85-96. [Crossref] [PubMed]
- Nitsche C, Maertin S, Scheiber J, et al. Drug-induced pancreatitis. Curr Gastroenterol Rep 2012;14:131-8. [Crossref] [PubMed]
- da Costa DW, Bouwense SA, Schepers NJ, et al. Same-admission versus interval cholecystectomy for mild gallstone pancreatitis (PONCHO): a multicentre randomised controlled trial. Lancet 2015;386:1261-8. [Crossref] [PubMed]
- Pinte L, Balaban DV, Băicuş C, et al. Non-alcoholic fatty pancreas disease - practices for clinicians. Rom J Intern Med 2019;57:209-19. [Crossref] [PubMed]
- Easler JJ, Kilian SJ, Kushnir VM, et al. Idiopathic recurrent acute pancreatitis (IRAP) and diminished parenchymal radiodensity on interval non-contrast CT scan (NCT). Is increased intraparenchymal pancreatic fat (IPF) content associated with irap? Gastroenterology 2014;146:S-620. [Crossref]
- Gonoi W, Akai H, Hagiwara K, et al. Pancreas divisum as a predisposing factor for chronic and recurrent idiopathic pancreatitis: initial in vivo survey. Gut 2011;60:1103-8. [Crossref] [PubMed]
- Bertin C, Pelletier AL, Vullierme MP, et al. Pancreas divisum is not a cause of pancreatitis by itself but acts as a partner of genetic mutations. Am J Gastroenterol 2012;107:311-7. [Crossref] [PubMed]
- Aslam M, Avanthi S, Ravikanth V, et al. Pancreas divisum increases the risk of idiopathic recurrent acute pancreatitis in the presence of cathepsin B L26V polymorphism. Clin Gastroenterol Hepatol 2017;15:154. [Crossref]
- Talukdar R, Aslam M, Reddy DN, et al. Pancreas Divisum Increases the Risk of Recurrent Acute Pancreatitis in Patients with rs12338 Polymorphism in the Cathepsin B Gene. Dig Dis Sci 2021;66:2283-90. [Crossref] [PubMed]
- Jang JW, Kim MH, Jeong SU, et al. Clinical characteristics of intraductal papillary mucinous neoplasm manifesting as acute pancreatitis or acute recurrent pancreatitis. J Gastroenterol Hepatol 2013;28:731-8. [Crossref] [PubMed]
- Elta GH. Sphincter of Oddi dysfunction and bile duct microlithiasis in acute idiopathic pancreatitis. World J Gastroenterol 2008;14:1023-6. [Crossref] [PubMed]
- van Brummelen SE, Venneman NG, van Erpecum KJ, et al. Acute idiopathic pancreatitis: does it really exist or is it a myth? Scand J Gastroenterol Suppl 2003;117-22. [Crossref] [PubMed]
- Ros E, Navarro S, Bru C, et al. Occult microlithiasis in 'idiopathic' acute pancreatitis: prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology 1991;101:1701-9. [Crossref] [PubMed]
- Shea JA, Berlin JA, Escarce JJ, et al. Revised estimates of diagnostic test sensitivity and specificity in suspected biliary tract disease. Arch Intern Med 1994;154:2573-81. [Crossref] [PubMed]
- Liu CL, Lo CM, Chan JK, et al. Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients. Gastrointest Endosc 2001;54:325-30. [Crossref] [PubMed]
- Guilmanova A, Sikorskaya E, Marino D, et al. A simple method for determining the etiology and treatment of idiopathic acute pancreatitis. Am J Gastroenterol 2011;106:S54. [Crossref]
- Umans DS, Hallensleben ND, Verdonk RC, et al. Recurrence of idiopathic acute pancreatitis after cholecystectomy: systematic review and meta-analysis. Br J Surg 2020;107:191-9. [Crossref] [PubMed]
- Paajanen H. Reply to Letter: Can Laparoscopic Cholecystectomy Prevent Recurrent Idiopathic Acute Pancreatitis? Ann Surg 2017;266:e95. [Crossref] [PubMed]
- Bianchi ML, De Luca L, Fabi MT, et al. The role of endoscopic ultrasonography in the etiological evaluation of idiopathic acute pancreatitis. United European Gastroenterol J 2013;1:A336-7.
- Boulay BR, Gordon SR, Gardner TB. The yield of endoscopic ultrasonography for determining an etiology in patients with idiopathic acute pancreatitis. Gastrointest Endosc 2009;69:AB248. [Crossref]
- Das K, Ray S, Bhattacharya D, et al. Natural history of Idiopathic Recurrent Acute Pancreatitis (IRAP): a prospective “real world” cohort study. Pancreatology 2019;19:S55. [Crossref]
- Garg PK, Tandon RK, Madan K. Is biliary microlithiasis a significant cause of idiopathic recurrent acute pancreatitis? A long-term follow-up study. Clin Gastroenterol Hepatol 2007;5:75-9. [Crossref] [PubMed]
- Hallensleben ND, Umans DS, Bouwense SA, et al. The clinical course and diagnostic work-up of idiopathic acute pancreatitis, a post-hoc analysis of a prospective multicenter observational cohort. Gastroenterology 2019;156:S-122. [Crossref]
- Kundu S, Conway J, Evans JA, et al. Endoscopic ultrasound (EUS) is highly effective in establishing an etiology in idiopathic pancreatitis. Gastrointest Endosc 2009;69:AB245. [Crossref]
- Liu CL, Lo CM, Chan JK, et al. EUS for detection of occult cholelithiasis in patients with idiopathic pancreatitis. Gastrointest Endosc 2000;51:28-32. [Crossref] [PubMed]
- Morris-Stiff G, Al-Allak A, Frost B, et al. Does endoscopic ultrasound have anything to offer in the diagnosis of idiopathic acute pancreatitis? JOP 2009;10:143-6. [PubMed]
- Norton SA, Alderson D. Endoscopic ultrasonography in the evaluation of idiopathic acute pancreatitis. Br J Surg 2000;87:1650-5. [Crossref] [PubMed]
- Ortega AR, Gómez-Rodríguez R, Romero M, et al. Prospective comparison of endoscopic ultrasonography and magnetic resonance cholangiopancreatography in the etiological diagnosis of "idiopathic" acute pancreatitis. Pancreas 2011;40:289-94. [Crossref] [PubMed]
- Pereira R, Eslick G, Cox M. Endoscopic Ultrasound for Routine Assessment in Idiopathic Acute Pancreatitis. J Gastrointest Surg 2019;23:1694-700. [Crossref] [PubMed]
- Rana SS, Bhasin DK, Rao C, et al. Role of endoscopic ultrasound in idiopathic acute pancreatitis with negative ultrasound, computed tomography, and magnetic resonance cholangiopancreatography. Ann Gastroenterol 2012;25:133-7. [PubMed]
- Saleem R, Raja O, Aujla UI, et al. Diagnostic yield of endoscopic ultrasound (EUS) in the evaluation of idiopathic pancreatitis. A single tertiary referral centre experience. Gut 2015;64:A55-6. [Crossref]
- Tepox-Padrón A, Bernal-Mendez RA, Duarte-Medrano G, et al. Utility of endoscopic ultrasound in idiopathic acute recurrent pancreatitis. BMJ Open Gastroenterol 2021;8:e000538. [Crossref] [PubMed]
- Valverde-López F, Ortega-Suazo EJ, Jiménez-Rosales R, et al. Predictingre lapse in idiopathic acute pancreatitis. is there a place for EUS? United European Gastroenterol J 2019;7:225.
- Vila JJ, Rubio E, Garaigorta M, et al. Prospective double blinded comparison of the diagnostic yield of endoscopic ultrasonography vs secretin enhanced cholangiomri in the etiological study of idiopathic acute pancreatitis. United European Gastroenterol J 2013;1:A10-1.
- Vila JJ, Vicuña M, Irisarri R, et al. Diagnostic yield and reliability of endoscopic ultrasonography in patients with idiopathic acute pancreatitis. Scand J Gastroenterol 2010;45:375-81. [Crossref] [PubMed]
- Wilcox CM, Seay T, Kim H, et al. Prospective Endoscopic Ultrasound-Based Approach to the Evaluation of Idiopathic Pancreatitis: Causes, Response to Therapy, and Long-term Outcome. Am J Gastroenterol 2016;111:1339-48. [Crossref] [PubMed]
- Yusoff IF, Raymond G, Sahai AV. A prospective comparison of the yield of EUS in primary vs. recurrent idiopathic acute pancreatitis. Gastrointest Endosc 2004;60:673-8. [Crossref] [PubMed]
- Smith I, Ramesh J, Kyanam Kabir Baig KR, et al. Emerging Role of Endoscopic Ultrasound in the Diagnostic Evaluation of Idiopathic Pancreatitis. Am J Med Sci 2015;350:229-34. [Crossref] [PubMed]
- Kirkegård J, Mortensen FV, Heide-Jørgensen U, et al. Predictors of underlying pancreatic cancer in patients with acute pancreatitis: a Danish nationwide cohort study. HPB (Oxford) 2020;22:553-62. [Crossref] [PubMed]
- Valverde-López F, Ortega-Suazo EJ, Wilcox CM, et al. Endoscopic ultrasound as a diagnostic and predictive tool in idiopathic acute pancreatitis. Ann Gastroenterol 2020;33:305-12. [Crossref] [PubMed]
- Wilcox CM, Seay T, Kim H, et al. Idiopathic pancreatitis: Endoscopic outcomes with long-term follow-up. Gastroenterology 2016;150:S700. [Crossref]
- Ahmed Ali U, Issa Y, Hagenaars JC, et al. Risk of Recurrent Pancreatitis and Progression to Chronic Pancreatitis After a First Episode of Acute Pancreatitis. Clin Gastroenterol Hepatol 2016;14:738-46. [Crossref] [PubMed]
- Di Leo M, Petrone MC, Mariani A, et al. Development of endosonographic signs suggesting chronic pancreatitis in patients with idiopathic recurrent acute pancreatitis. Dig Liver Dis 2013;45:S158. [Crossref]
- Boraschi P, Donati F, Cervelli R, et al. Secretin-stimulated MR cholangiopancreatography: spectrum of findings in pancreatic diseases. Insights Imaging 2016;7:819-29. [Crossref] [PubMed]
- Maydeo AP. Idiopathic recurrent pancreatitis: too many questions, too few answers. Gastrointest Endosc 2008;67:1035-6. [Crossref] [PubMed]
- Wan J, Ouyang Y, Yu C, et al. Comparison of EUS with MRCP in idiopathic acute pancreatitis: a systematic review and meta-analysis. Gastrointest Endosc 2018;87:1180-1188.e9. [Crossref] [PubMed]
- Avanthi SU, Ravi Kanth VV, Agarwal J, et al. Association of claudin2 and PRSS1-PRSS2 polymorphisms with idiopathic recurrent acute and chronic pancreatitis: A case-control study from India. J Gastroenterol Hepatol 2015;30:1796-801. [Crossref] [PubMed]
- Bishop MD, Freedman SD, Zielenski J, et al. The cystic fibrosis transmembrane conductance regulator gene and ion channel function in patients with idiopathic pancreatitis. Hum Genet 2005;118:372-81. [Crossref] [PubMed]
- Brand R, LaRusch J, Barmada MM, et al. Association of gamma-glutamyltransferase 1 gene (GGT1) polymorphisms with idiopathic chronic pancreatitis in the NAPS2 cohort. Gastroenterology 2011;140:S856. [Crossref]
- Castellani C, Gomez Lira M, Frulloni L, et al. Analysis of the entire coding region of the cystic fibrosis transmembrane regulator gene in idiopathic pancreatitis. Hum Mutat 2001;18:166. [Crossref] [PubMed]
- Cho SM, Shin S, Lee KA. PRSS1, SPINK1, CFTR, and CTRC Pathogenic Variants in Korean Patients With Idiopathic Pancreatitis. Ann Lab Med 2016;36:555-60. [Crossref] [PubMed]
- Garg PK, Khajuria R, Kabra M, et al. Association of SPINK1 gene mutation and CFTR gene polymorphisms in patients with pancreas divisum presenting with idiopathic pancreatitis. J Clin Gastroenterol 2009;43:848-52. [Crossref] [PubMed]
- Gomez-Lira M, Bonamini D, Castellani C, et al. Mutations in the SPINK1 gene in idiopathic pancreatitis Italian patients. Eur J Hum Genet 2003;11:543-6. [Crossref] [PubMed]
- Hamoir C, Pepermans X, Piessevaux H, et al. Clinical and morphological characteristics of sporadic genetically determined pancreatitis as compared to idiopathic pancreatitis: higher risk of pancreatic cancer in CFTR variants. Digestion 2013;87:229-39. [Crossref] [PubMed]
- Jalaly NY, Moran RA, Fargahi F, et al. An Evaluation of Factors Associated With Pathogenic PRSS1, SPINK1, CTFR, and/or CTRC Genetic Variants in Patients With Idiopathic Pancreatitis. Am J Gastroenterol 2017;112:1320-9. [Crossref] [PubMed]
- LaRusch J, Schneider A, Sun X, et al. Use of mathematical and statistical model predictions to identify a novel pancreas-specific class of CFTR variants linked to SPINK1 mutations and idiopathic chronic pancreatitis. Gastroenterology. 2010;138:S435. [Crossref]
- Maire F, Bienvenu T, Ngukam A, et al. Frequency of CFTR gene mutations in idiopathic pancreatitis. Gastroenterol Clin Biol 2003;27:398-402. [PubMed]
- Moran R, Yahyapourjalaly N, Kamal A, et al. Gene mutation testing for idiopathic pancreatitis: Predictors of diagnostic yield. Pancreatology 2016;16:S103. [Crossref]
- Ockenga J, Stuhrmann M, Ballmann M, et al. Mutations of the cystic fibrosis gene, but not cationic trypsinogen gene, are associated with recurrent or chronic idiopathic pancreatitis. Am J Gastroenterol 2000;95:2061-7. [Crossref] [PubMed]
- Oh HC, Kim MH, Do JH, et al. PRSS1 G208A and SPINK1 IVS3+2T>C were prevalent in korean patients with idiopathic or familial pancreatitis. Gastroenterology 2012;142:S619. [Crossref]
- Ockenga J, Stuhrmann M, Ballmann M, et al. Mutations of the cystic fibrosis gene, but not cationic trypsinogen gene, are associated with recurrent or chronic idiopathic pancreatitis. Am J Gastroenterol 2000;95:2061-7. [Crossref] [PubMed]
- Pelaez-Luna M, Robles-Diaz G, Canizales-Quinteros S, et al. PRSS1 and SPINK1 mutations in idiopathic chronic and recurrent acute pancreatitis. World J Gastroenterol 2014;20:11788-92. [Crossref] [PubMed]
- Pelletier AL, Bienvenu T, Rebours V, et al. CFTR gene mutation in patients with apparently idiopathic pancreatitis: lack of phenotype-genotype correlation. Pancreatology 2010;10:158-64. [Crossref] [PubMed]
- Ravikanth VV, Rupjyoti T, Mohsin A, et al. Genotype-Phenotype association of known variants conferring susceptibility to Idiopathic chronic pancreatitis. J Gastroenterol Hepatol 2019;34:395.
- Sarantitis I, Sheel A, Halloran C, et al. Symptom and diabetes onset in idiopathic pancreatitis: The role of SPINK1 p.N34S mutation. Pancreatology 2017;17:S104. [Crossref]
- Sarantitis I, Sheel A, Nicholson J, et al. The SPINK1 p.N34S variant is associated with early onset idiopathic recurrent acute pancreatitis progressing to chronic disease, but not with more rapid progression to other disease outcomes such as diabetes mellitus. Pancreatology 2018;18:S174-5. [Crossref]
- Sauter G, Sahin-Tóth M, Simon P, et al. Identification of a new N2K trypsinogen (PRSS1) mutation - possible association with idiopathic or hereditary pancreatitis. Pancreatology 2009;9:457-8.
- Shetty S, Krishnaveni J, Venkatakrishnan L, et al. A study of SPINK 1 mutation and clinical correlates in idiopathic recurrent acute pancreatitis. Indian J Gastroenterol 2013;32:A6.
- Shetty S, Sairam T, Janarthanan K, et al. Spink1 mutation in idiopathic recurrent acute pancreatitis-pilot study. J Clin Diagn Res 2018;12:OC15-7. [Crossref]
- Yahyapourjalaly N, Moran RA, Khashab MA, et al. Evaluation of factors associated with pathogenic PRSS1, SPINK1, CTFR, and/or CTRC gene mutation(S) in patients with idiopathic pancreatitis. Am J Gastroenterol 2016;111:S4-5. [Crossref]
Cite this article as: Cortés P, Raimondo M, Wallace MB, Bi Y. The role of endoscopic ultrasound and magnetic resonance cholangiopancreatography in the diagnosis of idiopathic recurrent acute pancreatitis: a narrative review. Dig Med Res 2021;4:69.