
Unusual endoscopic finding of a solitary ileocecal mass in mantle cell lymphoma—case report and literature review
Introduction
Extranodal manifestation of non-Hodgkin lymphoma (NHL) in the gastrointestinal tract is a common occurrence with an incidence of 5–20% (1). This presentation is attributed to secondary widespread nodal disease whereas primary gastrointestinal lymphomas are relatively rare accounting for 1–4% of all gastrointestinal malignancies (2). In general, the stomach is the most common site afflicted at 56.4% followed by the small intestines and ileocecal region which comprises around 16.4% of the cases (3).
Owing to the heterogenous nature of various NHL subtypes and their individual predilection for different gastrointestinal sites, there has been increasing efforts to better understand their behavior over the past two decades in terms of endoscopic, radiologic and histopathological findings (4). These modalities are continuously being refined to keep up with the technological sophistication on both diagnostic and therapeutic fronts with our focus here being on the field of diagnostic endoscopy (5).
Over the past decade, there were attempts to categorize endoscopic features of NHL into its morphological description. Iwamuro et al. classified these variants into protruded, fold thickening, multiple lymphomatous polyposis, ulcerative, superficial and mixed types and observed their characteristics in the various locations within the gastrointestinal tract. These lesions were mostly found in multiplicity with abnormal overlying mucosa for which biopsies were helpful in establishing a diagnosis. On the contrary, isolated lesions are uncommon and in addition to an endoscopically unremarkable mucosa, our case of solitary ileocecal valve swelling attributed to MCL is indeed rare and unique (6). In line with recent developments on gastrointestinal lymphoma, there is a constant need to describe and update on variant endoscopic findings to complement the currently established compendium of well-described morphological lesions. We believe that our case would alert clinicians in recognizing how atypical NHL may present amidst its subtle clinical picture alongside distinguishing them from other more common, benign pathologies.
We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/dmr-21-74).
Case presentation
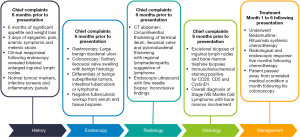
A 73-year-old man was referred to our hospital following three days of passing melenic stools in addition to experiencing dull epigastric pain. There were also symptoms of anemia that included lethargy, palpitations, reduced effort tolerance and dizziness. In addition, he reported significant appetite and weight loss over the past six months. His vital signs revealed blood pressure of 121/91 mmHg, pulse rate of 108 beats per minute, respiratory rate of 24 breaths per minute, temperature of 36.3 °C and an oxygen saturation of 98% on room air. He was clinically pale and mildly tachypneic. Initial systemic clinical examination was unremarkable.
Notable blood investigations revealed a severely low hemoglobin of 2.8 g/dL (normal range, 13.0–17.0 g/dL), total white count of 12.95×103/µL (normal range, 4.00×103–10.00×103/µL), platelet of 257×103/µL (normal range, 150×103–410×103/µL), albumin of 27 g/L (normal range, 34–48 g/L) and a lactate dehydrogenase of 261 U/L (normal range, 125–220 U/L) with otherwise normal liver function, renal profile and coagulation parameters. The infective screening, inflammatory panels and tumor markers were likewise unremarkable.
Following prompt resuscitation with fluids and blood products, our patient underwent a gastroscopy which revealed a large but clean based duodenal bulb ulcer (Forrest III) measuring 4 cm in size. Biopsies of the ulcer edge were benign and rapid urease test for Helicobacter pylori was negative. Colonoscopy performed two days later revealed a grossly swollen ileocecal valve with normal overlying mucosa (Figure 1). The neighboring ascending colon, cecum and appendiceal orifice were otherwise normal. Intubation into the terminal ileum was challenging owing to the compression from the swelling. Nevertheless, upon entry, the swelling could be tracked to extend contiguously by 2–3 mucosal folds proximally in an unusual serpentine fashion with preserved mucosal integrity on white light imaging (Figure 2). Closer inspection with narrow-band imaging (NBI) did not add further information aside from the clear demarcation between the swelling and rest of the ileum (Figure 3). Targeted biopsies of the abnormal ileocecal region revealed benign ileal tissues with Peyer’s patches. Owing to being an endemic region for tuberculosis (TB), in which intestinal manifestations are common, additional ileocecal biopsies were sent for TB workup namely tissue for acid fast bacilli (AFB), mycobacterium TB cultures and TB polymerase chain reaction (PCR). These were later found to be negative alongside an unremarkable chest X-ray and normal serum QuantiFERON-TB Gold. In addition to intestinal TB, concerns for gastrointestinal malignancy led us to perform a diagnostic reappraisal by reexamining our patient’s history and clinical findings. This eventually led to the discovery of bilateral enlarged inguinal lymph nodes that were initially missed. These lymph nodes measured 1–2 cm in size, were firm in consistency and non-tender.
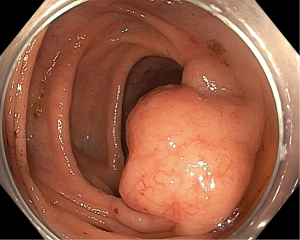
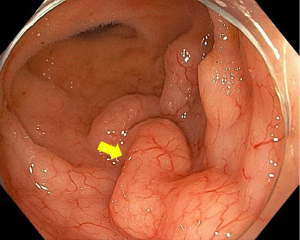
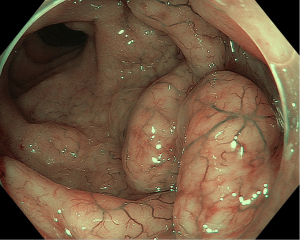
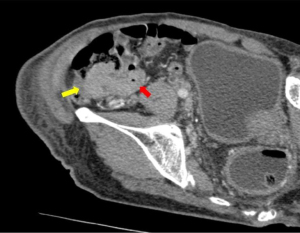
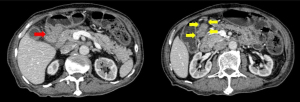
Following this, computed tomography (CT) scan of the abdomen revealed a homogenously enhancing circumferential wall thickening of the terminal ileum which was continuous with the grossly swollen ileocecal valve. The valve was seen to protrude inwardly, forming a solitary intraluminal mass which measured 3.8 cm × 3.4 cm × 5.0 cm (Figure 4). Enlarged mesenteric lymph nodes were also observed adjacent to the ileocecal region. There were otherwise no features of intestinal obstruction demonstrated. Distant to this site, there were concomitant pyloroduodenal wall thickening with regional peripancreatic, perigastric and periportal lymphadenopathy (Figure 5). Of note, the thickened walls and the enlarged lymph nodes shared similar degree of poor enhancement thereby proposing a unified pathology.
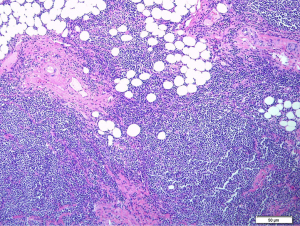
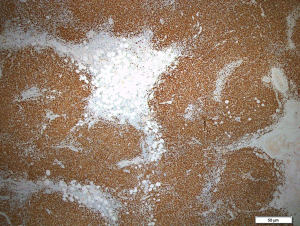
Initial endoscopic ultrasound with fine needle biopsy sampling of the periportal and peripancreatic nodes for histological interpretation were inconclusive. Hence, this led to an excisional biopsy of the enlarged inguinal lymph nodes revealing total architectural effacement of the lymph node by a nodular proliferation of medium-sized lymphoid cells. These cells exhibit even distribution of mild nuclei irregularity, condensed chromatin and inconspicuous nucleoli (Figure 6). Immunohistochemical staining further showed that these cells were diffusely positive for CD20 with co-expression of CD5 and CyclinD1 with a high proliferation index (Ki67) of >30% (Figure 7). The overall findings were consistent with a diagnosis of MCL. Additionally, a bone marrow trephine biopsy performed two weeks later confirmed marrow involvement. Collectively, this was in keeping with stage IVB MCL, and our patient was referred on to the hematology discipline for commencement of timely systemic chemotherapy.
Follow-up CT abdomen performed five months following chemotherapy revealed improvements in terms of abdominopelvic and inguinal lymphadenopathy resolution as well as size reduction of the ileocecal mass. Repeated colonoscopy a week following the CT abdomen likewise demonstrated complete normalization of the ileocecal region. There were also clinical improvements for which our patient gradually regained his weight and attained normalization of hemoglobin levels. The full timeline for our patient’s episode of care is summarized in pictorial format below (Figure 8).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Mantle cell lymphoma is a rare subtype of B-cell NHL occurring in 1 out of 200,000 people annually. It displays an aggressive behavior and generally presents at an advanced stage with poor prognosis (7). The molecular fingerprint of MCL is characterized by chromosomal translocation t(11;14)(q13;q32) which results in an overexpression of the CyclinD1 (CCND1) gene. Identification of the CCND1 protein on immunohistochemical study is thus key in discerning it from other B-cell lymphoma variants which would then guide subsequent treatment efforts (8).
As with the general predilection of NHL, the incidence of gastroduodenal MCL is more prevalent at 49% whereas that of colorectum ranges widely from 38–62% (9). In relevance to our case, MCL seems to possess a greater propensity for the terminal ileum and ileocecal region, which is in part attributed to the higher proportion of lymphoid tissues found here. Subsequent malignant transformation of these sites would then result in various gastrointestinal lesions described earlier (10,11). The dominant endoscopic appearance encountered is that of innumerable polypoidal lesions of varying sizes which are termed as multiple lymphomatous polyposis (12). This key finding alone accounts for approximately 77.3% of all intestinal lesions with less common variants being that of protruded type (18.2%) and superficial (4.5%) type. Solitary lesions on the other hand are highly unusual and the diagnostic challenge arises when one encounters a mass with endoscopically unremarkable mucosa like in our patient (13,14).
As with most occasions, histological corroboration is indispensable when there are uncertainties. Current literature reports favorable yield when these lesions manifest with loss of mucosal integrity such as ulceration or friability (14). This is a useful strategy to fall back on, and a good endoscopic and histologic correlation is all that is required to obtain a favorable diagnosis. On the flipside, lesions with endoscopically unremarkable mucosa remains a challenge in a third of cases where other forms of diagnostic tools are required depending on initial clinical presentation (14).
Unfortunately, the clinical manifestations of NHL are known to be vague and highly non-specific, which further adds to the diagnostic dilemma. With regards to gastrointestinal complaints alone, those that were commonly reported included abdominal pain, bloatedness, nausea, vomiting, anorexia and melena with symptoms attributed to anemia (15). These symptoms are not representative of gastrointestinal lymphoma alone as other more common gastrointestinal malignancy namely adenocarcinoma, and infection could also fit the clinical picture. It nevertheless sets a useful precedent on directing subsequent investigations, whether it be endoscopy or radioimaging thus allowing one to narrow down the list of differential diagnosis. On the contrary, severe clinical presentations reported with gastrointestinal lymphoma include signs of intestinal obstruction, ileocolonic intussusception and overt gastrointestinal bleeding which often signifies advanced disease warranting urgent surgical intervention alongside early oncology referral for systemic chemotherapy initiation (16).
Our case was unique in that the solitary involvement of the ileocecal valve gave off an unusual benign-looking appearance with smooth, glistening overlying normal mucosa. There was no ulceration or mucosal friability unlike recently reported solitary ileocecal lesions (17). Its contiguous extension into the terminal ileum in a serpiginous fashion rather than occurring in a circumferential or polypoidal pattern further adds to its eccentricity. Our initial impression was that this resembled an even rarer benign endoscopic finding, namely pneumocystis cystoid intestinalis, though these are generally soft to touch and collapse on biopsy sampling as they are filled with air (18). Gentle prodding with biopsy forceps revealed the ileocecal mass to be of firm consistency, and characterization with NBI was only helpful in demarcating the areas involved. Future role of NBI in lymphomas would require further exploration though current perspective is that it helps in lesion characterization and facilitating targeted biopsies for a better yield for which the latter was unhelpful in our case. This is despite evidence suggesting good yield in two-thirds of cases with endoscopically unremarkable mucosa. However, we need to be mindful that this evidence was nearly two decades old when endoscopic imaging was unlike that of the modern era in terms of visual clarity and the ability for on-demand magnification which allows for closer scrutiny (9,14).
As our case transpired, a thorough clinical reassessment performed for diagnostic clarification was pertinent in reformulating our line of thoughts. The identification of enlarged inguinal lymph nodes was on hindsight the most crucial piece of the puzzle that raised our suspicion for something more sinister and prompted us to pursue further relevant tests. The role of CT scan here is thus invaluable in further defining the endoscopic pathology while at the same time, delineate its’ origin and extent of involvement. Moreover, with the knowledge of inguinal lymphadenopathy, a complete evaluation of the lymphatic system is essential to screen for lymphoproliferative disorders. The common radiographic appearance of small bowel lymphoma described in the literature includes polypoidal, nodular, protruding, infiltrating, endoexoenteric form and mesenteric invasive type with an extraluminal mass (19). Ulceration and cavitation are occasionally encountered owing to the tumour size as they tend to outgrow their local blood supply. Other CT scan features include marked and symmetrical, circumferential bowel wall thickening with poor enhancement and homogenous attenuation (19). Though helpful, the cornerstone of obtaining a definitive diagnosis remains with histological assessment as some morphological variants could still resemble benign tumors. In this instance, the discovery of regional lymphadenopathy at multiple sites on CT imaging was advantageous as it offered a structured roadmap to guide subsequent selection of relevant diagnostic tools for tissue biopsy acquisition.
Following a conclusive diagnosis, our patient was started on the Bedamustine-Rituximab (BR) chemotherapy regime, for which he responded positively, despite being diagnosed at an advanced stage. Briefly, the BR regime is an effective first-line option with favorable response rates and outcomes in patients who are elderly and ineligible for autologous stem cell transplant (20). This highlights the importance of maintaining a high index of clinical suspicion followed by detailed examination, especially in a patient presenting with non-specific complaints in addition to unusual endoscopic lesions. Despite good outcomes, it was unfortunate that our patient succumbed to an unrelated illness due to severe sepsis with multiorgan failure secondary to bronchopneumonia a month after his colonoscopy. This negated the opportunity to observe his progression-free survival alongside disease recurrence following systemic chemotherapy.
Conclusions
Gastrointestinal involvement of NHL and specifically MCL remains a diagnostic enigma owing to the heterogenous endoscopic appearance and unfamiliarity amongst endoscopists especially when unusual variants are encountered. Modern and sophisticated endoscopes alongside an increase in performing diagnostic procedures translate to more of these pathologies being increasingly detected on a regular basis. This would warrant the ongoing need to continuously bridge the gap of knowledge between novel findings with their associated pathology in hopes of improving timely diagnosis, leading to an overall better outcome. Nevertheless, the progressive advent of technology should not replace the very essence of basic history taking and clinical assessment. Omitting the findings of inguinal lymphadenopathy would have led to unnecessary, or worse, no follow-up investigations that would presage a dire outcome for our patient because of diagnostic delay. Thus, the emphasis on clinical reappraisal is pertinent in yielding previously overlooked information that would be useful in guiding relevant diagnostic tests.
In summary, our case highlights a unique endoscopic presentation of MCL which could have been easily passed off as benign following a negative biopsy had it not been for the subsequent discovery of enlarged inguinal lymph nodes. We trust that our findings would serve as a useful addition in the field of diagnostic endoscopy and broaden insights into the spectrum of unique findings in MCL. Further to this, we propose that future research directions should focus on developing a widely accepted and descriptive classification for endoscopic lesions related to MCL. The classification needs to be sufficiently simple to adopt in routine clinical practice and supported by complementary diagnostic features such as NBI, endoscopic ultrasound, radiology and histology.
Acknowledgments
We wish to express our appreciation by acknowledging our team of gastrointestinal nurses who assisted us and provided valuable insights and feedback during the colonoscopy. They are AMO Valentine Lesley Philiminus, RN Betty Singa and RN Ellesa Rabius from Queen Elizabeth Hospital, Kota Kinabalu Sabah, Malaysia. We would also like to express our gratitude to Dr. Sandhya Rajaintharan for her efforts in providing us with the histopathology images.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/dmr-21-74
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/dmr-21-74). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer 1972;29:252-60. [Crossref] [PubMed]
- Howell JM, Auer-Grzesiak I, Zhang J, et al. Increasing incidence rates, distribution and histological characteristics of primary gastrointestinal non-Hodgkin lymphoma in a North American population. Can J Gastroenterol 2012;26:452-6. [Crossref] [PubMed]
- Chen Y, Chen Y, Chen S, et al. Primary Gastrointestinal Lymphoma: A Retrospective Multicenter Clinical Study of 415 Cases in Chinese Province of Guangdong and a Systematic Review Containing 5075 Chinese Patients. Medicine (Baltimore) 2015;94:e2119. [Crossref] [PubMed]
- Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol 2011;17:697-707. [Crossref] [PubMed]
- Vetro C, Romano A, Amico I, et al. Endoscopic features of gastro-intestinal lymphomas: from diagnosis to follow-up. World J Gastroenterol 2014;20:12993-3005. [Crossref] [PubMed]
- Iwamuro M, Okada H, Kawahara Y, et al. Endoscopic features and prognoses of mantle cell lymphoma with gastrointestinal involvement. World J Gastroenterol 2010;16:4661-9. [Crossref] [PubMed]
- Dantoc MM, Eslick GD, Adams SS, et al. Gastrointestinal Mantle Cell Lymphoma-A Tale of Two Endoscopies. J Gastrointest Cancer 2012;43:S20-4. [Crossref] [PubMed]
- Nunes G, Sequeira P, Fernandes V. Mantle cell lymphoma of the cecum. Rev Esp Enferm Dig 2019;111:333-4. [Crossref] [PubMed]
- Romaguera JE, Medeiros LJ, Hagemeister FB, et al. Frequency of gastrointestinal involvement and its clinical significance in mantle cell lymphoma. Cancer 2003;97:586-91. [Crossref] [PubMed]
- Koch P, del Valle F, Berdel WE, et al. Primary gastrointestinal non-Hodgkin's lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001;19:3861-73. [Crossref] [PubMed]
- Bairey O, Ruchlemer R, Shpilberg O. Non-Hodgkin's lymphomas of the colon. Isr Med Assoc J 2006;8:832-5. [PubMed]
- Hirata N, Tominaga K, Ohta K, et al. A case of mucosa-associated lymphoid tissue lymphoma forming multiple lymphomatous polyposis in the small intestine. World J Gastroenterol 2007;13:1453-7. [Crossref] [PubMed]
- Daniel F, Assi HI, Karaoui W, et al. A Single Mass Forming Colonic Primary Mantle Cell Lymphoma. Case Rep Gastrointest Med 2016;2016:2561507. [Crossref] [PubMed]
- Salar A, Juanpere N, Bellosillo B, et al. Gastrointestinal involvement in mantle cell lymphoma: a prospective clinic, endoscopic, and pathologic study. Am J Surg Pathol 2006;30:1274-80. [Crossref] [PubMed]
- Kim YH, Lee JH, Yang SK, et al. Primary colon lymphoma in Korea: a KASID (Korean Association for the Study of Intestinal Diseases) Study. Dig Dis Sci 2005;50:2243-7. [Crossref] [PubMed]
- Negrean V, Graur F, Moiş E, et al. Ileocecal Obstruction Due to B-cell Non-Hodgkin Lymphoma. Chirurgia (Bucur) 2016;111:71-3. [PubMed]
- Matsueda K, Toyokawa T, Sakata M, et al. Mantle Cell Lymphoma with a Single Protruding Lesion as the Cause of Intussusception. Intern Med 2018;57:1751-5. [Crossref] [PubMed]
- Azzaroli F, Turco L, Ceroni L, et al. Pneumatosis cystoides intestinalis. World J Gastroenterol 2011;17:4932-6. [Crossref] [PubMed]
- Balthazar EJ, Noordhoorn M, Megibow AJ, et al. CT of small-bowel lymphoma in immunocompetent patients and patients with AIDS: comparison of findings. AJR Am J Roentgenol 1997;168:675-80. [Crossref] [PubMed]
- Visco C, Chiappella A, Nassi L, et al. Rituximab, bendamustine, and low-dose cytarabine as induction therapy in elderly patients with mantle cell lymphoma: a multicentre, phase 2 trial from Fondazione Italiana Linfomi. Lancet Haematol 2017;4:e15-23. [Crossref] [PubMed]
Cite this article as: Chiam KH, Peter J, Ho CV, Muthukaruppan R. Unusual endoscopic finding of a solitary ileocecal mass in mantle cell lymphoma—case report and literature review. Dig Med Res 2021;4:77.


