Unique challenges of endotherapeutics in malignant lower gastrointestinal bleeding in a patient with COVID-19 pneumonia—case report and literature review
Introduction
Lower gastrointestinal bleeding (LGIB) constitutes 20–30% of all major gastrointestinal bleeding cases for which diverticular disease, ischemic colitis, colonic angioectasia and hemorrhoidal bleeding comprises the main etiologies (1). Colorectal tumours however, contribute up to 17% of LGIB cases and may present with either occult bleeding, melena or haematochezia (2,3). This is dependent on the location of the tumour with right-sided colonic cancers presenting often with occult blood loss, melena and iron deficiency anaemia whereas patients with left-sided colonic cancers are more prone to come in with haematochezia (4).
Thankfully, the majority of tumour-related LGIB is slow enough to avert the need for endoscopic intervention though acute bleeding with hemodynamic instability can still infrequently occur (3). Under normal circumstances, patient stabilization and subsequent surgical intervention remains the mainstay of treatment to address this issue. Nevertheless, there are a minority of instances where endoscopic intervention are required to buy time when appropriate surgical measures are not immediately available.
Our aim of this case is to describe the available endoscopic hemostatic modalities and challenges encountered in managing a case of malignant LGIB with limited resources in light of the recent limitations and technical burdens imposed by the COVID-19 pandemic.
We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/dmr-21-56).
Case presentation
A 59-year-old man was referred to our hospital for isolation following 2 days complain of low-grade fever, cough and flu-like symptoms while attending a haemodialysis session. This was in light of a recent COVID-19 outbreak in the district prison where our patient was an inmate. The relatively crowded holding cells raised concern that he could have come into contact with a COVID-19 patient and was thus triaged as a person under investigation. On arrival to the hospital, preliminary clinical assessment revealed his blood pressure to be 160/70 mmHg, pulse rate of 95 beats per minute, respiratory rate of 16 breaths per minute, temperature of 38 °C and oxygen saturation of 100% on room air. His comorbidities included end-stage renal failure, hypertension, ischemic heart disease and gouty arthritis. Other relevant history taking was not remarkable at this point of time. He was pale on clinical examination but otherwise comfortable with warm peripheries and good pulse volume. Respiratory findings were positive for coarse crepitations over at the right lower zones of the chest while other systems examinations were unremarkable. Based on the initial assessment, a chest X-ray confirmed the presence of right middle lobe consolidation of the lung in keeping with bronchopneumonia. Subsequent to this, his nasopharyngeal swab was positive for COVID-19 and he was treated for stage 3 COVID-19 pneumonia.
Relevant blood investigations revealed severe normocytic normochromic anaemia with haemoglobin counts of 5.1 g/dL (normal range, 13.0–17.0 g/dL). Other notable results included a total white blood count of 6.79×103/µL (normal range, 4.00–10.00×103/µL), platelet of 304×103/µL (normal range, 150–410×103/µL), urea of 9.7 mmol/L (normal range, 3.0–9.2 mmol/L), creatinine of 474 µmol/L (normal range, 63.6–110.5 µmol/L), serum albumin of 25 g/L (normal range, 35–50 g/L), alanine transaminase of 44 U/L (normal range, 0–55 U/L) and a normal coagulation profile.
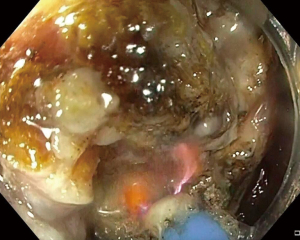
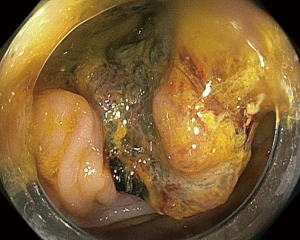
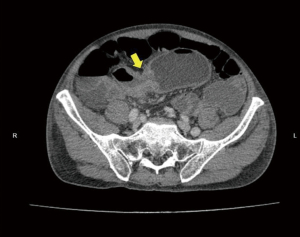
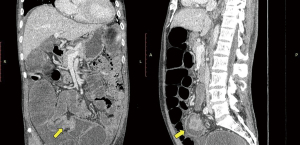
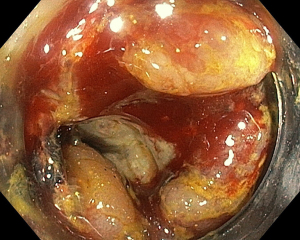
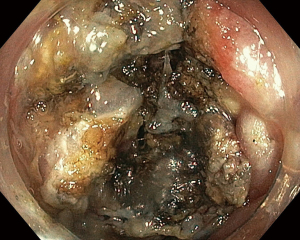
During the third day of admission, he complained of haematochezia for which further history revealed that he has been passing out blood mixed with stools intermittently for the past 1 month. There was also passage of mucus per rectally and altered bowel habits for the past 3 months. He also complained of intermittent non-specific generalized abdominal pain which resolves upon defecation but otherwise could not appreciate any abdominal swelling. There was significant history of weight and appetite loss. Both digital rectal examination and proctoscopy were unremarkable. Hence colonoscopy was scheduled for the next day and this revealed a circumferential, fungating tumour within the rectosigmoid colon with significantly narrowed lumen estimated to be 3–4 mm in size (Figure 1). As there was no active bleeding seen, no endoscopic intervention was forthcoming. Targeted biopsies taken were confirmatory for adenocarcinoma and a staging computed tomography (CT) scan performed showed a localized, circumferential thickening at the rectosigmoid junction traversing across a length of 5.0 cm with intraluminal narrowing (Figures 2,3). There were no proximal bowel dilatation and no distant metastases. He was then managed with blood transfusion and remained asymptomatic until 10 days later when he developed massive haematochezia with hemodynamic instability. His haemoglobin dropped from 9.3 to 6.4 g/dL and an urgent colonoscopy was scheduled following patient stabilization. Bedside colonoscopy revealed multiple bleeding points from the ulcerated and necrotic tumour surface making it difficult to localize the exact bleeding origin (Figure 4). Endoscopic ablation with argon plasma coagulation (APC) monotherapy was used to treat the large bleeding tumour surface owing to the absence of other therapeutic modalities (Figures 5,6). Our subsequent strategy was to repeat the procedure should rebleeding occur until he can be transferred out for definitive surgery.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Worldwide, the incidence of LGIB shows a gradual increasing trend with age peaking at the seventh decade of life. Though the annual incidence remains low at 0.03%, the growing elderly population and increasing use of newer oral anticoagulants are causes for concern (1). The presentation of LGIB is varied, ranging from minor blood loss that can be managed expectantly to torrential, life-threatening bleeding that require urgent intervention.
Endoscopic management for LGIB are not too dissimilar from that of upper gastrointestinal (GI) bleeding (UGIB) with regards to currently available hemostatic devices. The complexity however arises with GI-tumour related bleeding due to its heterogeneous morphology as compared to benign etiologies (5). In relevance to our case, we aim to discuss the various endoscopic accessories available and focus on the challenges and limitations of each available method.
The most common therapeutic modality is adrenaline which is typically injected around the bleeding site in four-quadrant points (6). This is to create a vascular tamponade effect alongside local vasoconstriction leading to a thrombogenic state which would slow down the bleeding rate and allowing clear visualization for a second modality to be applied to achieve hemostasis (6,7). Adrenaline is thus useful as an adjunctive therapy and not as a standalone monotherapy due to its temporary local effect (8). Unfortunately, this method was not favourable in our case as we were not able to manoeuvre beyond the malignant stricture to visualize and intervene at its proximal extent.
Next in line are thermal devices which are classified into contact and noncontact thermal techniques. Currently available contact thermal accessories in our centre include coagulation forceps and the bipolar hemostasis catheters with built-in flushing capabilities. Other recognized modalities not available in our centre are heater and monopolar probes. These devices share a common similarity in dealing with focal bleeding points where mild contact pressure is applied against the area of interest before thermal energy is delivered (9). It works best in a targeted manner and is generally not beneficial when dealing with tumour bleeding as these tend to be multifocal and widespread. Several studies looking into its efficacy reported high 30-day rebleeding rates ranging from 33–80% despite initial successful hemostasis (10,11).
On the flipside, APC is a safe, simple and cost-effective noncontact thermal therapeutic modality which provides an alternative strategy for managing large bleeding areas (12). This approach utilizes ionized argon gas to deliver thermal energy resulting in localized tissue necrosis and coagulation. When used appropriately, the thermal effect on the mucosal depth is limited superficially (2–3 mm) and thus avoid the risk of intestinal perforation (5). As tumour morphology differs from one to another in various locations, large controlled studies with APC are limited. One retrospective review reported excellent rates of hemostasis in all of their patients using APC with or without adjuvant adrenaline injection. The reported rebleeding rates were 30% and could be managed with blood transfusion and angioembolization treatment (13). The usefulness of APC in our case is the capability to treat a wide surface area covering multiple bleeding points without the need for probe contact on the tissue. This is useful as tumour-related bleeding is known to arise from a combination of factors such as surface ulceration, neovascularization and local blood vessel invasion (14,15). To ensure optimal hemostasis outcomes, we incorporated both pulsed (Effect 2, 20–30 Watts, 2.0 L/min) and forced APC (20–40 Watts, 2.0 L/min) modes on the ERBE VIO 200 D electrosurgical unit (VIO® 200 D, Erbe Medical UK Ltd., Leeds, UK) in order to reach both superficial and deeper tissue bleeding sources. The probe was placed 5–6 mm away from the tumour tissue and controlled firing performed on the bleeding site until coagulation occurred. This was carried out in a systematic proximal to distal, clockwise fashion to allow for better visualization of subsequent bleeding targets.
Recently, the role of endoscopic management of bleeding GI tumours took a positive turn with the introduction of hemostatic powder namely, Hemospray (Hemospray®, Cook Medical, Winston-Salem, NC, USA). This is a highly absorptive inorganic mineral powder that interacts with blood, forming a mechanical barrier to prevent further bleeding while at the same time do not cause tissue alteration via dessication or coagulation (16). Initial reports for benign LGIB etiologies were optimistic with 88–100% of immediate bleeding control and relatively low rebleeding rates ranging from 3–13% (17). Similar outcomes for malignant GI bleeding were also seen in a recent pilot study where immediate hemostasis was achieved in 87.7% of patients at index endoscopy. Moreover, the 180-day rebleeding rates were three times lower than those who received conventional hemostatic therapy (18). The appealing feature of Hemospray is the noncontact and atraumatic application which is favourable for bleeding tumours that are often friable and ooze easily upon contact making retreatment an inevitability. Further to this, its application does not require an en face position thus making it simple to adopt in routine endoscopic practices. It works well both as a primary modality and salvage therapy though larger, well-designed trials focusing purely on LGIB tumours are needed to support this claim (19). Unfortunately, the timing of our case coincided with the global recalling of Hemospray devices due to technical issues leaving us with no other alternatives except for APC. On hindsight, Hemospray would be an ideal salvage therapy in our case should APC fail.
In our patient, the decision to perform endoscopic hemostasis with APC was made following close consultation with the surgical team. As our patient was being treated for COVID-19 and would require 2 more weeks of isolation, the primary intent was to buy some time before he could be subjected for further management. The task of securing hemostasis was an arduous one as we had to deal with tumour friability and the inadvertent APC-induced tissue trauma. The latter is a known complication due to the large spraying surface area of the catheter that may result in unwanted collateral damage. As our hospital is not equipped with angioembolization services and definitive surgical treatment was not possible at this stage, our capabilities were strictly constrained to sole endoscopic intervention.
Aside from limitations in endoscopic modalities, other unique issues encountered that needs highlighting included ergonomic challenges and reduced tactile sensation on scope handling due to the mandatory wearing of multiple layers of personal protective equipment, limited working space in the isolation cubicle, time-limited rechargeable mobile respirator apparatus, communication breakdown from bulky headgears and mental exhaustion which could all take a heavy toll on optimal endoscopic performance. The donning and doffing procedures are burdensome with equipment and patient preparation taking up a lengthy time leaving behind a shorter interval for the endoscopic procedure before the respirator’s battery is drained. If the procedure is incomplete, the whole endoscopy team would have to repeat the whole safety preparatory steps to re-enter. Though these are unavoidable compounding factors, overlooking them would certainly presage a negative outcome for the patient.
Against all odds, our efforts were rewarded as his haematochezia subsided and we managed to curtail both inotropic requirements and blood transfusion postprocedurally. He was subsequently discharged after a month in the hospital and returned 6 weeks later for an elective laparoscopic anterior resection procedure. The final histopathology of the resected tumour was reported as a pT3N0M0 moderately differentiated adenocarcinoma.
Conclusions
Malignant LGIB cases profound enough to necessitate hemostatic intervention are a minority, and our case scenario examines the unprecedented issue that occurred during the COVID-19 pandemic where endoscopic intervention poses a challenge. This would have been somewhat different if hemostatic powders had been readily available given the overall promising prospects for lower rebleeding rates and ease of use. Despite this shortcoming, we were able to circumvent the problem with endoscopic APC to temporarily control the bleeding colonic tumour while awaiting definitive intervention. We need to be aware that despite APC’s temporizing hemostatic benefits, there remains the risk of significant rebleeding which could be addressed with repeat endoscopic procedure (13,20). However, the COVID-19 pandemic has presented us with unique limitations to endoscopic practice as described earlier, some of which may affect endoscopic performance and impact overall patient outcomes. These issues need to be highlighted and outrightly addressed as they could be effectively mitigated through prior strategic planning, robust teamwork collaboration and multidisciplinary management.
Acknowledgments
We wish to express our appreciation by acknowledging our team of gastrointestinal nurses who assisted us and provided valuable insights and feedback during the colonoscopy. They are RN Nelson Jerry Daniel Tadam, RN Mark James Gaban, RN Betty Singa and RN Norjanah Kamil from Queen Elizabeth Hospital, Kota Kinabalu, Malaysia. Likewise, we would also like to acknowledge our gastroenterology fellow, Dr. Cha Chee Tan for co-managing the patient throughout his stay in the hospital.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/dmr-21-56
Peer Review File: Available at https://dx.doi.org/10.21037/dmr-21-56
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/dmr-21-56). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- ASGE Standards of Practice Committee. The role of endoscopy in the patient with lower GI bleeding. Gastrointest Endosc 2014;79:875-85. [Crossref] [PubMed]
- Longstreth GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol 1997;92:419-24. [PubMed]
- Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am 2005;34:643-64. [Crossref] [PubMed]
- Barnert J, Messmann H. Diagnosis and management of lower gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol 2009;6:637-46. [Crossref] [PubMed]
- Ofosu A, Ramai D, Latson W, et al. Endoscopic management of bleeding gastrointestinal tumors. Ann Gastroenterol 2019;32:346-51. [PubMed]
- Hirao M, Masuda K, Asanuma T, et al. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc 1988;34:264-9. [Crossref] [PubMed]
- Fujishiro M, Iguchi M, Kakushima N, et al. Guidelines for endoscopic management of non-variceal upper gastrointestinal bleeding. Dig Endosc 2016;28:363-78. [Crossref] [PubMed]
- Kanai M, Hamada A, Endo Y, et al. Efficacy of argon plasma coagulation in nonvariceal upper gastrointestinal bleeding. Endoscopy 2004;36:1085-8. [Crossref] [PubMed]
- Wong Kee Song LM, Baron TH. Endoscopic management of acute lower gastrointestinal bleeding. Am J Gastroenterol 2008;103:1881-7. [Crossref] [PubMed]
- Savides TJ, Jensen DM, Cohen J, et al. Severe upper gastrointestinal tumor bleeding: endoscopic findings, treatment, and outcome. Endoscopy 1996;28:244-8. [Crossref] [PubMed]
- Loftus EV, Alexander GL, Ahlquist DA, et al. Endoscopic treatment of major bleeding from advanced gastroduodenal malignant lesions. Mayo Clin Proc 1994;69:736-40. [Crossref] [PubMed]
- Herrera S, Bordas JM, Llach J, et al. The beneficial effects of argon plasma coagulation in the management of different types of gastric vascular ectasia lesions in patients admitted for GI hemorrhage. Gastrointest Endosc 2008;68:440-6. [Crossref] [PubMed]
- Thosani N, Rao B, Ghouri Y, et al. Role of argon plasma coagulation in management of bleeding GI tumors: evaluating outcomes and survival. Turk J Gastroenterol 2014;25:38-42. [Crossref] [PubMed]
- Sheibani S, Kim JJ, Chen B, et al. Natural history of acute upper GI bleeding due to tumours: short-term success and long-term recurrence with or without endoscopic therapy. Aliment Pharmacol Ther 2013;38:144-50. [Crossref] [PubMed]
- Heller SJ, Tokar JL, Nguyen MT, et al. Management of bleeding GI tumors. Gastrointest Endosc 2010;72:817-24. [Crossref] [PubMed]
- Sung JJ, Luo D, Wu JC, et al. Early clinical experience of the safety and effectiveness of Hemospray in achieving hemostasis in patients with acute peptic ulcer bleeding. Endoscopy 2011;43:291-5. [Crossref] [PubMed]
- Mourad FH, Leong RW. Role of hemostatic powders in the management of lower gastrointestinal bleeding: A review. J Gastroenterol Hepatol 2018;33:1445-53. [Crossref] [PubMed]
- Chen YI, Wyse J, Lu Y, et al. TC-325 hemostatic powder versus current standard of care in managing malignant GI bleeding: a pilot randomized clinical trial. Gastrointest Endosc 2020;91:321-328.e1. [Crossref] [PubMed]
- Chan SM, Lau JYW. Is hemospray the ultimate answer to malignant GI bleeding? Gastrointest Endosc 2020;91:329-31. [Crossref] [PubMed]
- Johnstone C, Rich SE. Bleeding in cancer patients and its treatment: a review. Ann Palliat Med 2018;7:265-73. [Crossref] [PubMed]
Cite this article as: Chiam KH, Muthukaruppan R. Unique challenges of endotherapeutics in malignant lower gastrointestinal bleeding in a patient with COVID-19 pneumonia—case report and literature review. Dig Med Res 2021;4:57.