Robotic primary bariatric surgery
Introduction
As the prevalence of obesity is rising and becoming a worldwide problem, there is an increased demand for bariatric surgery. In patients with high body mass index (BMI), large sized livers, thick abdominal walls and large amount of visceral fat, performing bariatric surgery becomes demanding. This is secondary to technical and ergonomic difficulties including appropriate exposure and reconstruction (1). This holds especially true for patients with a BMI ≥50 kg/m2 and BMI >60 kg/m2. Term superobese (SO) and super-super obese has been used to describe them respectively. This sub set of obese patients pose specific challenge with much higher anesthetic risk and comorbidities. In addition to that, exposure is decreased significantly with reduced working space and torqueing adds significantly to ergonomic difficulties secondary to thick abdominal wall (2,3). Moreover, the surgeons may encounter extremely difficult ergonomic positions, which can possibly be very distressing and career shortening for them. The surgeons have been on the lookout for ergonomically favorable ways to improve the patient outcomes and surgical technique while decreasing size of incisions and complications.
The da VinciTM surgical robot was introduced by Intuitive Surgical in the United States of America in year 2000, Inc., providing an attractive surgical alternative. It was also cleared by Food and Drug Administration for laparoscopic surgery at the same time. With time, use of the robotic platform has increased, especially for complex abdominal operations including bariatric as well as revisional bariatric procedures.
Cadiere et al. reported the first case of use of robotics in bariatric surgery in 1999 and it has been evolving ever since (4). Major advantage provided by robotic system is three-dimensional vision, improved degrees of freedom and better precision by taking care of physiological tremors (5,6). It enables better tissue dissection and suturing. In improves surgical ergonomics by nullifying excessive torque placed on ports due to thick abdominal wall and thus reduces port site trauma (7). However, robotic bariatric surgery is perceived to be associated with increased operative time as well as cost. With time, it is being noticed that set up time of robotic platform is getting reduced. With induction of new players in the market, instruments and other associated things are expected to get cheaper (8).
Operating room set up for robotic bariatric procedures
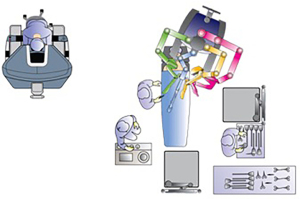
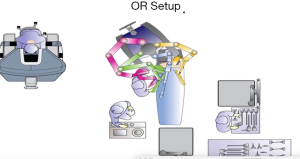
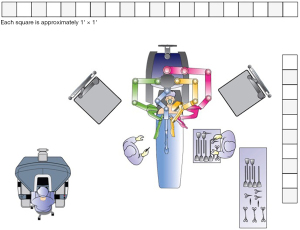
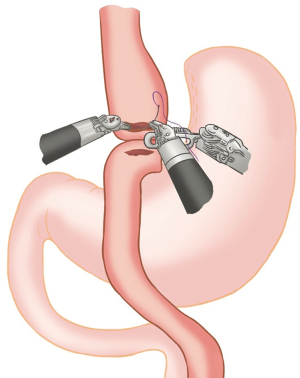
Though various types of bariatric procedures are technically different, set up of surgical space is more or less same for all (Figure 1). There is some variation in operating room setup depending on whether da VinciTM Si or Xi surgical platform is being used.
The surgeon’s console is placed in a way that operating surgeon can directly view the surgical field, and the communication between the surgeon and scrub team is easy. The video monitor and robot tower are positioned towards the head end of the patient. The anesthesia cart and instrument table are set up on one side of the patient. In the Si system, the patient cart is docked either from head end or parallel docking ensuring adequate clearance of the robot arms from the operating room table or the patient. In Xi system, the boom can move and thus the patient cart can come from one side of the patient. Target anatomy, camera port and central column all lie in the same straight line. Depending on the specific bariatric procedure, sone changes in the orientation of the robotic system may be required.
In Xi platform, the camera is 8 mm, instruments are longer, with universal arms where a camera can be docked onto any arm. The arms are mounted on a rotating beam, and docking is easier. With table motion technology, the operating table can be moved even when the robotic arms are docked. The master console essentially remains very similar to Si platform.
Various robot assisted bariatric procedures will be discussed subsequently.
Robotic Roux-en-Y gastric bypass
Roux-en-Y gastric bypass is the most well accepted procedure used for morbidly obese subject and majority of robotic bariatric literature available is related to it (9,10). Data show excellent results both in terms of improvement/remission of comorbid conditions as well as improvement in terms of weight (11). This procedure involves two anastomoses [gastrojejunostomy (GJ) and Jejuno-jejunostomy]. Hand sewn anastomosis is proven to be superior and is considered to be associated with fewer complications compared to a stapled one. With availability of robotic platform and its advantages described above, performing this surgery becomes simpler (12).
Our technique
RYGB at our center is done in a totally robotic fashion with gastro-jejunostomy being hand sewn. The details pertaining to instruments and procedure have been described subsequently.
Instrumentation
Robotic instruments (da Vinci® Surgical System (Intuitive Surgical, Sunnyvale, CA):
- R1- Ultrasonic shears: 5 mm.
- Large needle driver: 8 mm.
- Monopolar hook: 8 mm.
- R2- Cadiere forceps: 8 mm.
- R3- Double fenestrated grasper: 8 mm.
Position
Patient is to be placed in supine position with left arm tucked by the side. The robotic cart will come in from patient’s head end or left shoulder and anesthesia cart will be placed on right side of patient. Bedside surgeon is present on right side of patient with scrub nurse and sterile table placed on right side. 20° of reverse Trendelenburg position is given to the patient after padding under both knees and adequately strapping the patient. Placing a urinary catheter is optional.
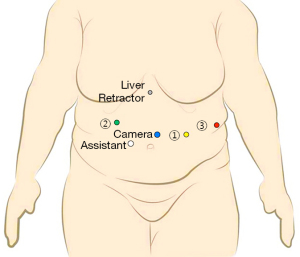
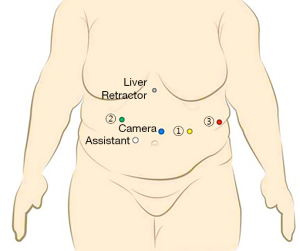
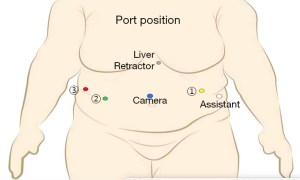
Port placement: (Figure 2 )
Pneumoperitoneum created at palmer’s point using Veress needle (closed technique).
Using optical trocar, camera port is inserted slightly to left of midline, approximately 20 cm below xiphisternum. All other ports placed under vision to avoid any inadvertent injury.
After insufflation, the external markings and landmarks on skin would change. So we recommend to mark and plan the port position after pneumoperitoneum has been achieved.
- C→ Camera port placed in between umbilicus and Xiphisternum approximately 20 cm below the later, and keeping it slight to left of midline.
- R2→ In right mid-clavicular line, 20 cm from Xiphisternum in arc like fashion.
- A (Assistant Port, 12 mm)→Between port C and port R2 at least 8 cm apart.
- R1→ in left mid clavicular line, 20 cm from xiphisternum.
- R3→ in left anterior axillary line approximately at the same level of camera.
- Nathanson liver retractor is used for retracting left lobe of liver by placing a 5 mm port below xiphisternum.
Technique
Diagnostic laparoscopy and Bowel marking
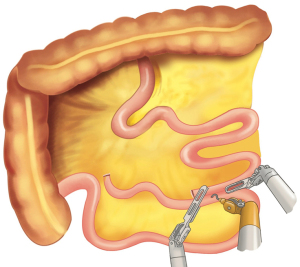
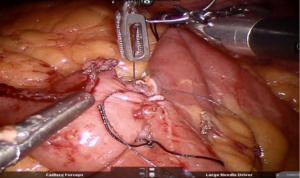
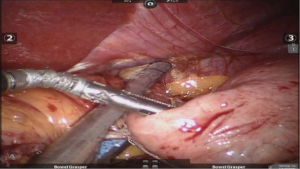
After placing the ports and the liver retractor, a thorough diagnostic laparoscopy is done. Omentum and transverse colon is lifted up to expose small bowel, and the duodeno-jejunal flexure/ligament of Trietz is identified. 75 cm of small bowel is measured from DJ flexure and it is hitched to stomach near lesser curvature at two sites using non absorbable sutures (Figure 3). Here, one should take care to keep proximal loop (biliopancreatic limb) on left side of patient. Beyond that point, another 100 cm of small bowel is measured distally and marked using non-absorbable suture at two sites 5 cm apart keeping proximal stitch small and distal stitch longer.
Robot docking
Patient cart brought in from head end. The central column of the patient cart, target anatomy (stomach in this case) and the camera port, all lie in single straight line. Two mobile video monitors are placed on either side of the patient to enable the assistants to easily watch and help at every step of procedure, to provide ergonomic comfort.
Operative room set-up (Figure 4 )
Gastric pouch formation
Dissection is started using following instruments in three arms:
- R1-Harmonic 5 mm long.
- R2-cadiere forceps.
- R3-Double fenestrated grasper.
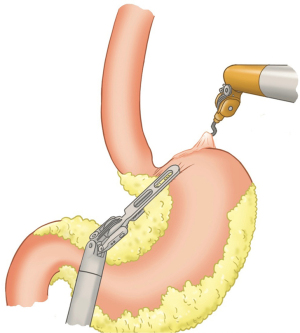
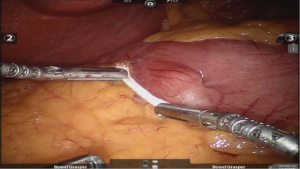
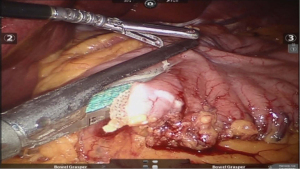
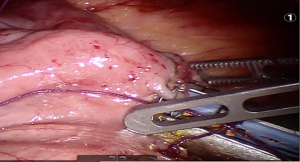
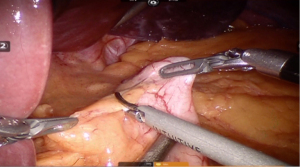
Using peri-gastric dissection, a small gastric pouch of capacity 20–30 mL is created. To start with, Phrenoesophageal membrane is divided using monopolar energy or ultrasonic shear while fundus of stomach is retracted caudally. Perigastric dissection is commenced by division of Gastrohepatic ligament between the first and second division of left gastric vessel and lesser sac is entered. It is done with the help of Harmonic scalpel in R1while stomach is being retracted laterally using R3. Care is taken to avoid injury to Vagus while entering lesser sac (Figures 5,6).
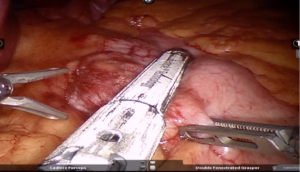
After delineating and entering lesser sac a 60-mm blue/tan cartridge is used by the surgeon on patient side to start division of stomach horizontally (Figures 7,8).
Adhesiolysis is done in lesser sac, lysing posterior stomach attachments as one progress cranially towards the angle of his. A couple of vertical stapler firings are usually required using 60-mm blue/purple cartridge to further divide the stomach and a bougie is placed prior to this to size the pouch. This completes gastric pouch creation (Figure 9).
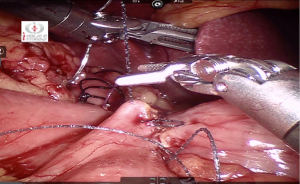
Gastro-jejunal anastomosis
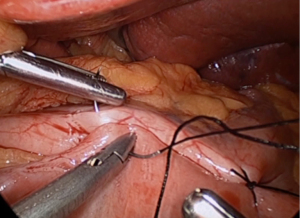
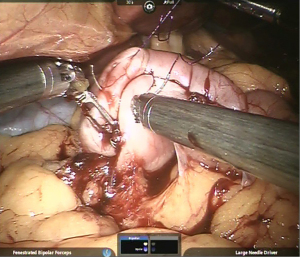
Using barbed suture, imbrication of vertical limb of gastric pouch is continued as fourth layer of Gastro-Jejunostomy (Figure 10).
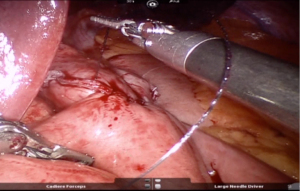
Gastrostomy & Enterotomy made using Monopolar hook/harmonic shears and stoma widened using harmonic shears in R2 creating a stoma length of 2–2.5 cm approximately (Figure 11).
Using a new barbed suture, third layer (posteriorly) started from left side, towards right side. The same suture is reversed at the opposite end (right) to form the 2nd layer of anastomosis (anteriorly).
Anterior-most layer completed using another barbed suture thus completing the anastomosis (Figure 12).
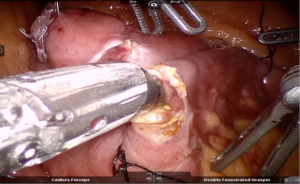
Jejuno-jejunal anastomosis
Window created in Jejunum mesentery just proximal to Gastro-jejunostomy (towards left side) and jejunum transacted using white/tan-60 mm cartridge thus creating a biliopancreatic limb of 100 cm (Figure 13).
Roux limb measured for 100 cm from Gastro-jejunostomy till we reach the previously placed marking sutures. Enterotomies made at 100 cm mark and in the biliopancreatic limb.
Jejuno-jejunostomy (side to side) is made using blue/tan 60 mm cartridge) (Figures 14,15).
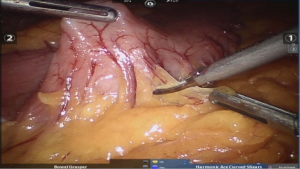
Enterotomy closed in single layer using non-absorbable sutures from below upwards in a continuous fashion (Figure 16).
Closure of mesenteric defects
The mesenteric defect (defect between small bowel mesenteries) is closed using non-absorbable suture 2-0 below upwards.
The Peterson defect (defect between alimentary limb and transverse colon) is closed using continuous non -absorbable suture.
Upper GI endoscopy/methylene blue is used to rule out any intraluminal bleeding or leak (intra-operative leak test). A Jackson-pratt drain is placed which is removed after 48 hrs.
Literature review
There have been nine published series of significance comparing outcomes of RRYGB versus laparoscopic RYGB (LRYGB) (13-21). These studies represent the entire literature on RRYGB and its outcomes. For each study, we review demographic data including number of patients, age, preoperative BMI, OR time, type of GJ (sutured or stapled), length of hospital stay, overall complication rate, leak rate, GJ stricture rate and mortality. There were a total of 3,337 patients in these 9 studies with 1,381 in a robotic arm and 1,956 in the laparoscopic arm. The mean age in RRYGB and LRYGB group was 43.3 years and 42.4 years, respectively. Average BMI was 45.8 in RRYGB patients and 46.7 in LRYGB patients.
The mean OR time was 211.9 min in robotic arm versus 185.1 min in the laparoscopic arm. Three studies report a significantly shorter operative time in a robotic group (13,15,19), while four studies report a significantly longer operative time in a robotic arm (16,17,20,21). This longer operative time in robotic arm may be due to hand sewn GJ in robotic group that was stapled in laparoscopic group in six out of nine studies. The average length of stay was 5 and 7.1 days in robotic and laparoscopic arm respectively. Three studies found a statistically significant shorter hospital stay in the robotic arm (15,20,21), while one study found a significantly longer stay in the robotic arm (19).
Complication rates overall were 13.3% in LRYGB while it was 12.2% in RRYGB group. Significant difference in the rate of complications was noted favoring RRYGB in the study by Buchs et al. (11.6% vs. 16.1%) (21). Studies of Snyder et al. and Buchs et al. demonstrate lower leak rates of 0.9% in RRYGB as compared to 1.6% in LRYGB group (14,21). As per their study, GJ stricture rate was 3.1% in a robotic arm and 3.2% in the laparoscopic arm. Benizri et al. found a significantly higher GJ stricture rate for a robotic arm (19). Mortality rates in both the groups was almost the same of 0.05%.
Of all the published studies, there is only one prospectively randomized trial by Sanchez et al. (13). The other studies are either comparative studies, case series, retrospective or prospective analyses. In majority of these studies, there is a surgeon skill bias as majority of the surgical teams are utilizing either of the two techniques with very few performing both laparoscopic and robotic surgeries with equal competency. But large comparative studies and systematic reviews do offer some tendencies for robotic bariatric surgery (22-25).
A series of 1100 RYGB’s have been published by Tieu et al. It is one of the largest series robot assisted RYGB that has been published have published (26). Average operative time in this series was 155 minutes without any conversions and very few complications. It included 2 cases of pulmonary embolism (0.19%), 3 cases of deep venous thrombosis (0.27%), 1 case of GJ anastomotic leak (0.09%), and 9 cases of staple line bleeding (0.82%).
A metaanalysis was conducted by Wang et al. for comparison of surgical outcomes RRYGB and LRYGB in terms of safety and efficacy (27). Study included 1 RCT and 18 CCTs in which total number of RRYGB were 5,532 as compared to 172,234 LRYGB. They found no significant differences in terms of major complications between the two techniques. They were also comparable in terms of other factors apart from operative time and cost that was higher in RRYGB.
Studies show significant difference in the learning curve of two techniques. It appears to be distinctly lower in RRYGB. Buchs et al. found that if a surgeon is proficient in laparoscopy even though not in bariatric procedures, number of cases required to overcome the learning curve is 14 (12) while 75–100 number of cases are considered to be the learning curve for LRYGB (28,29). Major complications in first 100 cases that has been performed using robotic platform was studied by Yu et al. and it did not reveal any leak and one reoperation (30). Abundant published data is available in favor of robot assisted RYGB in terms of learning curve.
Robotic sleeve gastrectomy
Sleeve gastrectomy is undoubtedly the most commonly performed bariatric procedure throughout the world at present. It has gained popularity due to its technical simplicity, shorter duration, lesser morbidity and comparable surgical outcomes. It is especially so in Indian sub-continent because of high prevalence of a vegetarian population, who tend to choose a restrictive procedure rather than a malabsorptive one. While performing Sleeve gastrectomy Hiatal (left crus) dissection is required for mobilizing fundus entirely. It is one of the major steps of SG for surgical success. Safe and precise dissection in the area is of utmost importance as any inadvertent injury in this area is most susceptible to leak. Gastric sleeve formation requires a long staple line making it a potential site for bleeding or leak. When we compare RRYGB with LRYGB, former definitely have an edge for oversewing the staple line as well as the hiatal dissection secondary to its endowrist action. The first robotic sleeve gastrectomy, as part of the BPD/DS, was performed in 2000 (31), but the first series of standalone robotic sleeve gastrectomies was reported in 2011 (32). We are performing robotic sleeve gastrectomies since 2012 and our technique is detailed below.
Technique
Instrumentation
The following robotic instruments are used for a RSG in a da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA).
- Large needle driver
- Ultrasonic shears
- Atraumatic bowel grasper forceps
- Laparoscopic/Robotic staplers
Patient positioning & OR setup (Figure 17 )
The patient is positioned supine with arms by the side. After cleaning and draping, an orogastric tube placed to decompress stomach. Patient is secured in place by placing straps appropriately to prevent slipping. A body warming blanket is placed in situ.
Port position and docking
We use closed technique for creating pneumoperitoneum using Veress needle from palmer’s point and insuffulate it to 15 mm of Hg. Port positions are marked and distance between two ports is measured after creating pneumoperitoneum, as it has been noted that port positions are not as desired if marked beforehand. This holds especially true for morbidly obese secondary to their pendulous abdominal wall. Trocar position and their placement is important in robotic surgery to avoid clashing of external arm. It is generally recommended to maintain a minimum inter-trocar distance of 8–10 cms. It has been observed that intra peritoneal distance is much less than that measured in between trocars over body surface. It order to maintain optimal distance between instruments and avoid external arm clashing, placing trocars at a maximal possible distance over body surface is advisable. Pre-emptive analgesia (0.5% bupivacaine) infiltration is done prior to insertion of all ports.
After achieving pneumoperitoneum, camera port is inserted using visiport approximately 20 cm below xiphisternum and slightly to the left of midline. Remaining robotic trocars are placed after this (Figure 18).
- R1: placed in left mid clavicular line approx. 20 cm from xiphisternum
- R2: placed in right hypochondrium in mid clavicular line taking care of liver size as well
- R3: placed in left flank at the level of camera port
- Assistant port (12 mm diameter) is placed in between camera port and R2 with a distance of at least 10 cm from both of them.
- A 5 mm epigastric port is made and used for placing Nathanson liver retractor for retracting left lobe of liver.
To make the surgical procedure ergonomically sustainable, these port positions can be modified based on body habitus of the patient. A diagnostic laparoscopy is always done after placement of all the ports. This is done to detect any other pathological condition present and to rule out in inadvertent injury during port placement as well.
The robotic platform is placed towards left shoulder of patient (parallel docking in Si system).
Parallel docking gives more room to the anesthetist, as well as endoscopist for intra-operative gastroscopy. The third arm of the robot comes from left side of the patient. The assistant surgeon stands by the side for complimentary maneuvers (i.e., suction, stapling, retraction etc.). With the launch of robotic staplers, the stapling can also be done from the console itself, giving the operating surgeon greater control on the surgical procedure.
Operative technique
Position of the patient is kept reverse Trendelenberg’s at the start of the procedure. After induction, a Gastric Calibration Tube (GCT) of 38 Fr is used to decompress stomach and kept in situ to facilitate gastric sleeve formation. Caution is taken while placing GCT as inadvertent injuries have been noted and reported. We place a nasogastric tube inside the GCT to be able to efficiently suck out the gastric secretions, which otherwise tend to pool in the wide end of GCT. Care is taken to empty out all the gastric secretions, especially from the fundus, so as to facilitate hiatal dissection. The GCT is then withdrawn into esophagus before starting the gastrolysis.
Pylorus is identified and a 5 cm umbilical tape is used to measure distance of 5cms from pylorus along greater curvature where gastrolysis is begun using an energy source (Figure 19).
Stomach is elevated using bowel grasper in R2, gastrocolic ligament is retracted laterally using bowel grasper in R3 and gastrolysis is performed with the help of energy source in R1 (Figure 20).
Dissection is done remaining juxtastomach to avoid injury to gastroepiploic vessels. As we proceed towards fundus of the stomach, short gastric vessels are encountered that are meticulously divided. Complete mobilization of fundus is ensured as we approach left crus and the later is meticulously defined further. While approaching and working near left crus, bougie is ensured to be present in esophagus as if beyond GE junction, it may interfere with dissection near hiatus. Hiatus hernia if identified, should be repaired to avoid gastroesophageal reflux. Distal portion of gastrocolic ligament is divided until 4–5 cm from pylorus. Any adhesions present between the stomach and anterior surface to the pancreas are taken down at this point.
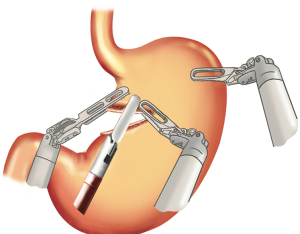
After ensuring complete mobilization of stomach, GCT is pushed forward gently along the lesser curvature into first part of duodenum by the anesthetist. This movement should be under vision and be guided by the surgeon using bowel graspers. Coordination between surgeon and anesthetist is important in the process and while negotiating the pylorus. This position of bougie along the lesser curve into the duodenum helps to a large extent in the formation of an even sleeve. To maintain correct orientation, traction is applied along the greater curvature of stomach by the primary surgeon at the console while articulating staplers are used through assistant port for creation of sleeve. For creation of sleeve, 1st reload used is usually green, following which multiple blue reloads are used and we try not be snug to GCT in this process. Utmost precaution is taken to prevent uneven staple line or spiraling (Figure 21).
Stapling at incisura angularis done carefully to prevent narrowing. Transection is continued towards the angle of his along the lateral edge while maintaining lateral symmetrical traction. A straight staple line is formed without any spiral either anteriorly or posteriorly to prevent a functional obstruction. Finally, transection is done at angle of HIS (Figure 22), avoiding an acute angle at GE junction.
Imbrication of staple line is done keeping bougie in place using barbed 2-0 running suture as shown in figure below (Figure 23).
We always do a check gastroscopy with saline immersion all the cases so as to rule out any leak, bleeding or obstruction.
Literature review
Five studies have been published, which focus on robotic sleeve gastrectomy (RSG) (32-36). Diamantis et al. published a feasibility study that included 19 patients who underwent RSG (33). Their average operative time was 95.5 min without any reported complication. In a study published by Ayloo et al., 30 robotic with 39 laparoscopic SG procedures were compared and they did not find any difference between these two in terms of complication, length of hospital stay and loss of excess body weight at the end of 1 year (32). However, they reported significantly longer OR time for RSG (135 vs. 114 min), this was attributed to oversewing of staple line that was done only in RSG.
Vilallonga et al. reported that the learning curve of performing RSG is over by 20 cases (34). He also published a large comparative series on SG including 100 patients each in robotic and laparoscopic arms (35). They found that OR time was significantly longer in a robotic group by 12 min but did not find any significant difference in the complication rate. They found that leaks occurred only in those patients in whom they did not over-sew the staple line, but used a buttress material. They concluded that RSG is a good stepping stone to robotic gastric bypass and RBP. Romero et al. published their experience of 134 RSG cases and compared it with descriptive results of a systematic review of laparoscopic SG (n=3,148) (36). The OR time was significantly higher by 12 min (P=0.006), whereas hospital stay was lower by 1 days in a robotic group (P≤0.005). Leaks were found in 0 RSG versus 1.97% laparoscopic SG (P=0.101); strictures in 0 versus 0.43% (P=0.447); bleeding in 0.7% versus 1.21% (P=0.594); and mortality in 0 versus 0.1% (P=0.714), respectively.
A study was performed at our center to compare results of RSG involving 78 subjects in morbidly obese (MO) and 34 in super obese (SO) category. The mean time taken for the procedure was 116.3 min with docking time of 8.9. We did not find any difference in the OR time, duration of hospitalization or complications between the two groups. So, as per our study, we can conclude that use of Robots attenuates the challenges that are normally faced while performing surgeries in SO, enabling surgeon to perform bariatric surgery in all categories of patient with equal ease and precision without altering the duration of surgery.
Robotic biliopancreatic diversion with duodenal switch
One of the most technically demanding bariatric procedures to perform is biliopancreatic diversion with duodenal switch (BPD/DS). It involves formation of a gastric sleeve in which pylorus is kept intact and sleeve is divided beyond pylorus. 2nd step involves bypassing majority of small bowel and distal ileum is anastamosed to gastric sleeve (duodenum) in a Roux-en-Y fashion. The first robotic BPD/DS was performed in October, 2000 soon after the introduction of the da VinciTM system (31).
Technique
Patient position and OR set up (Figure 24 )
Patient in supine position with 20° reverse Trendelenberg tilt. OR set up is more or less same as RRYGB.
Port position (Figure 25 )
Three robotic arms with one assistant trocar and Nathanson’s liver retractor placed.
Diagnostic laparoscopy and bowel marking
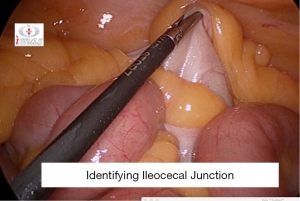
After a through diagnostic laparoscopy is performed, 1st step is to identify the ileocaecal junction (ICJ), following which small bowel is measured proximally for 250 cms and is marked at 100 and 250 cm to facilitate prepare Roux limb of 150 cm and common channel of 100 cm (Figure 26).
Marked bowel at 250 cm is hitched to anterior abdominal wall. Retro duodenal tunnel is meticulously dissected following which transaction is done at the level of D1 (Figure 27).
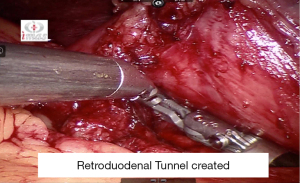
Duodeno-ileostomy is done using 3-0 barbed absorbable suture in a hand sewn fashion at 250 cm mark using 3-0 absorbable barbed suture (Figure 28).
Common limb is made of 100 cm by performing Jejuno-illeostomy at 100 cm mark using 60 mm blue/tan cartridge (Figure 29). Enterotomy closed using absorbable suture in two layers.
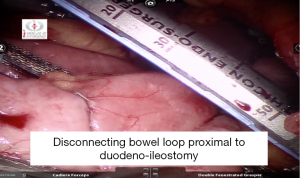
Loop of bowel divided just proximal to duodeno-illeostomy (Figure 30).
Intraoperative endoscopy/Methylene blue dye test is done to rule out any leak. Non absorbable sutures are used for closure of all the mesenteric defects.
Sudan et al. reported the outcomes, learning curve and technique of robotic biliopancreatic diversion with duodenal switch (RBPDDS) (31,37,38). They published experience of 47 patients who underwent this procedure. The mean BMI in their series was 45 kg/m2 with average age of patients being 38 years. Their initial median OR time was 514 min, which decreased to 379 min in last 10 patients. There were four leaks, three conversions and no mortality in the series. The literature reveals learning time for RBPDDS to be more as compared to other robotic bariatric procedures i.e., around 50 cases following which operative time and complication reduce significantly (37).
Antanavicius et al. (39) in 2016 reported their technique of Total Robotic Biliopancreatic Diversion with Duodenal Switch using robotic staplers with an experience of more than 200 patients with good results. The same author in 2020 conducted a retrospective analysis of 304 consecutive bariatric patients who had robotic or robotic-assisted BPD/DS from December 2008 to February 2018 from a single operating surgeon (40). Thirty 30- and 90-day complication and readmission rates were analyzed. There was no anastomotic leak and no mortality reported.
Robotic adjustable gastric banding
The first robotic bariatric procedure performed after introduction of robotic platform was Adjustable gastric banding (AGB) (4). Technically it is considered the simplest of all bariatric procedures. Number of AGB has decreased tremendously all over the world because of low efficacy and high revision/complication rate associated with it. Limited literature is available evaluating robot assisted AGB and the available data does not show any significant benefit over laparoscopic AGB (41-43). Largest study done for robotic AGB by Edelson et al. involved comparative analysis involving 287 and 120 patients in robotic and laparoscopic AGB respectively (44). It did not reveal any significant difference between the two groups either in terms of operative time, length of hospital stay or outcomes. However, they did notice a longer duration of surgery in laparoscopic AGB by 12 minutes in SO group of patients. As of now, robot is used mostly for managing complications and revising gastric band to another weight loss procedure.
Robotic revisional bariatric procedures
Revisional bariatric procedures are increasing owing to problems of weight regain or as a complication of any other bariatric procedure. These are difficult and complex situations in which use of robotics hold a great potential. The anatomy in these situations is distorted which along with adhesions present a challenge to the surgeons. Complication rates of laparoscopy in these situations have been high with leak rates of 13.2% and mortality of 2% (45). There is a couple of studies available on RBP that show better outcomes with robot assistance in these challenging cases (46,47).
Snyder et al. reviewed 99 cases that were revised using robot assistance at one centre (46). The mean BMI decreased from 44.8 to 29 kg/m2 after 3 years. However, the complication rate was not great as the reduction in BMI. Almost one fourth of the patients required readmission within 90 days and complication rate was to the tune of 17%. There was no reported hemorrhage, leak or mortality in the series, which is promising considering the high incidence of these complications in these situations. Buchs et al. compared the outcomes of RBP performed by robotic (n=11), laparoscopic (n=21) and open (n=28) method (47). The study did not show any complication in robotic arm whereas complication rates in laparoscopic and open technique were 14.3% and 10.7% respectively. At the same time RBP performed by robot took longer to perform (352 vs. 270 vs. 250 min, in robotic, laparoscopic and open groups respectively). There were fewer conversions in robotic group (0 vs. 14.3% for laparoscopy) and a significantly shorter hospital stay (6 vs. 8 vs. 9 days, respectively). Study by Bindal et al. included 32 patients with robotic RYGB done as a RBP (48). There was no leak, hemorrhage or anastomotic stricture reported in the series, which is a pretty good outcome considering the higher complication rate in revisional bariatric procedures.
Discussion
This article highlights the safety and feasibility of robot and clearly shows that it can be routinely used for bariatric procedures. Significant literature is available revealing its post-operative outcomes being better to laparoscopic bariatric procedures in terms of complications like anastomotic leak, stricture or bleeding. The advantage of robotics is perceived much more in challenging situations like RBP. However, in order to graduate to these advanced procedures, one has to move a step by step, starting with RSG and moving on to RRYGB and RBP.
Any advanced form of surgery requires a well-trained team as every member of the team is equally important. This holds especially true important in case of Robotic Bariatric Procedures. Be it scrub nurse or bedside surgeon, all have well defined roles. If the nursing staff involved is not trained well, surgery is not streamlined in appropriate fashion leading to unnecessary delay or resources. With the principal surgeon at the console, bedside surgeon is involved in all the stapling work in case when robotic staplers are not used. He/she should be competent enough to handle any emergency that may arise during the procedure. In case the assistant surgeon is inadequately trained, situation can be difficult to handle.
The learning curve of RRYGB has also been shown to be shorter as compared to LRYGB (12). Entire team should ideally undergo a formal training programme prior to involvement in Robotic bariatric practice. With time, the entire team gains rich experience along with the chief surgeon pertaining to various aspects of surgery which ultimately results in shorter OR time and better outcomes.
The robotic system offers other advantaged like improved ergonomics and lesser fatigue of the surgeon. It has been highlighted in a large number of studies that poor ergonomics during laparoscopic surgery leads to early exhaustion and musculoskeletal injury. This is more common in SO patients (49). Robotic platform is especially useful in these situations and offers the advantage of more degrees of freedom, which are particularly beneficial while performing difficult dissection and sutured anastomosis.
Cost has always been the center of debate whenever robotic surgery is being discussed. When direct cost is being analysed, it always appears to be higher (50). However, when the overall cost is taken into consideration it doesn’t appear so. This has been clearly shown by a study conducted by Hagen et al. where they also took into consideration the complications and readmission (18). Thus, the overall or total cost as per their study was lower for RRYGB when compared to LRYGB. Hand-sewn anastomosis in place of use of stapler during robotic procedures also decreases the cost.
Robotic system provides for a digital interface in between the patient and the surgeon. That interface itself has a huge potential with artificial intelligence and machine learning coming in. There are newer robotic systems from different companies coming in, which is providing much needed thrust to this modality. Looking at the basic concept of computer-assisted navigational surgery, robotics provides an enabling platform in between surgeon and the patient. With the advent of newer technologies in robotics like fluorescence, integration of images, virtual and augmented reality, tele-surgery, single site platforms, natural orifice surgery and haptic feedback, we believe that it will provide an empowering tool to the surgeons, which can potentially change the way surgery is practiced today.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alfredo Daniel Guerron) for the series “Advanced Laparoscopic Gastric Surgery” published Digestive Medicine Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: https://dx.doi.org/10.21037/dmr-21-33). The series “Advanced Laparoscopic Gastric Surgery” was commissioned by the editorial office without any funding or sponsorship. All authors have no other conflicts of interest to declare.
Ethical Statement: All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wilson EB, Sudan R. The evolution of robotic bariatric surgery. World J Surg 2013;37:2756-60. [Crossref] [PubMed]
- Parikh MS, Shen R, Weiner M, et al. Laparoscopic bariatric surgery in super-obese patients (BMI>50) is safe and effective: a review of 332 patients. Obes Surg 2005;15:858-63. [Crossref] [PubMed]
- Gagner M, Gumbs AA, Milone L, et al. Laparoscopic sleeve gastrectomy for the super-super-obese (body mass index >60 kg/m(2)). Surg Today 2008;38:399-403. [Crossref] [PubMed]
- Cadiere GB, Himpens J, Vertruyen M, et al. The world's first obesity surgery performed by a surgeon at a distance. Obes Surg 1999;9:206-9. [Crossref] [PubMed]
- Talamini MA, Chapman S, Horgan S, et al. A prospective analysis of 211 robotic-assisted surgical procedures. Surg Endosc 2003;17:1521-4. [Crossref] [PubMed]
- Bindal V, Bhatia P, Kalhan S, et al. Robot-assisted excision of a large retroperitoneal schwannoma. JSLS 2014;18:150-4. [Crossref] [PubMed]
- Cadière GB, Himpens J, Vertruyen M, et al. Evaluation of telesurgical (robotic) NISSEN fundoplication. Surg Endosc 2001;15:918-23. [Crossref] [PubMed]
- Nakadi IE, Mélot C, Closset J, et al. Evaluation of da Vinci Nissen fundoplication clinical results and cost minimization. World J Surg 2006;30:1050-4. [Crossref] [PubMed]
- Schauer PR, Ikramuddin S. Laparoscopic surgery for morbid obesity. Surg Clin North Am 2001;81:1145-79. [Crossref] [PubMed]
- Buchwald H, Williams SE. Bariatric surgery worldwide 2003. Obes Surg 2004;14:1157-64. [Crossref] [PubMed]
- Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683-93. [Crossref] [PubMed]
- Buchs NC, Pugin F, Bucher P, et al. Learning curve for robot-assisted Roux-en-Y gastric bypass. Surg Endosc 2012;26:1116-21. [Crossref] [PubMed]
- Sanchez BR, Mohr CJ, Morton JM, et al. Comparison of totally robotic laparoscopic Roux-en-Y gastric bypass and traditional laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis 2005;1:549-54. [Crossref] [PubMed]
- Snyder BE, Wilson T, Leong BY, et al. Robotic-assisted Roux-en-Y Gastric bypass: minimizing morbidity and mortality. Obes Surg 2010;20:265-70. [Crossref] [PubMed]
- Ayloo SM, Addeo P, Buchs NC, et al. Robot-assisted versus laparoscopic Roux-en-Y gastric bypass: is there a difference in outcomes? World J Surg 2011;35:637-42. [Crossref] [PubMed]
- Park CW, Lam EC, Walsh TM, et al. Robotic-assisted Roux-en-Y gastric bypass performed in a community hospital setting: the future of bariatric surgery? Surg Endosc 2011;25:3312-21. [Crossref] [PubMed]
- Scozzari G, Rebecchi F, Millo P, et al. Robot-assisted gastrojejunal anastomosis does not improve the results of the laparoscopic Roux-en-Y gastric bypass. Surg Endosc 2011;25:597-603. [Crossref] [PubMed]
- Hagen ME, Pugin F, Chassot G, et al. Reducing cost of surgery by avoiding complications: the model of robotic Roux-en-Y gastric bypass. Obes Surg 2012;22:52-61. [Crossref] [PubMed]
- Benizri EI, Renaud M, Reibel N, et al. Perioperative outcomes after totally robotic gastric bypass: a prospective nonrandomized controlled study. Am J Surg 2013;206:145-51. [Crossref] [PubMed]
- Myers SR, McGuirl J, Wang J. Robot-assisted versus laparoscopic gastric bypass: comparison of short-term outcomes. Obes Surg 2013;23:467-73. [Crossref] [PubMed]
- Buchs NC, Morel P, Azagury DE, et al. Laparoscopic versus robotic Roux-en-Y gastric bypass: lessons and long-term follow-up learned from a large prospective monocentric study. Obes Surg 2014;24:2031-9. [Crossref] [PubMed]
- Markar SR, Karthikesalingam AP, Venkat-Ramen V, et al. Robotic vs. laparoscopic Roux-en-Y gastric bypass in morbidly obese patients: systematic review and pooled analysis. Int J Med Robot 2011;7:393-400. [Crossref] [PubMed]
- Fourman MM, Saber AA. Robotic bariatric surgery: a systematic review. Surg Obes Relat Dis 2012;8:483-8. [Crossref] [PubMed]
- Kim K, Hagen ME, Buffington C. Robotics in advanced gastrointestinal surgery: the bariatric experience. Cancer J 2013;19:177-82. [Crossref] [PubMed]
- Toro JP, Lin E, Patel AD. Review of robotics in foregut and bariatric surgery. Surg Endosc 2015;29:1-8. [Crossref] [PubMed]
- Tieu K, Allison N, Snyder B, et al. Robotic-assisted Roux-en-Y gastric bypass: update from 2 high-volume centers. Surg Obes Relat Dis 2013;9:284-8. [Crossref] [PubMed]
- Wang L, Yao L, Yan P, et al. Robotic Versus Laparoscopic Roux-en-Y Gastric Bypass for Morbid Obesity: a Systematic Review and Meta-Analysis. Obes Surg 2018;28:3691-700. [Crossref] [PubMed]
- Schauer P, Ikramuddin S, Hamad G, et al. The learning curve for laparoscopic Roux-en-Y gastric bypass is 100 cases. Surg Endosc 2003;17:212-5. [Crossref] [PubMed]
- Oliak D, Ballantyne GH, Weber P, et al. Laparoscopic Roux-en-Y gastric bypass: defining the learning curve. Surg Endosc 2003;17:405-8. [Crossref] [PubMed]
- Yu SC, Clapp BL, Lee MJ, et al. Robotic assistance provides excellent outcomes during the learning curve for laparoscopic Roux-en-Y gastric bypass: results from 100 robotic-assisted gastric bypasses. Am J Surg 2006;192:746-9. [Crossref] [PubMed]
- Sudan R, Puri V, Sudan D. Robotically assisted biliary pancreatic diversion with a duodenal switch: a new technique. Surg Endosc 2007;21:729-33. [Crossref] [PubMed]
- Ayloo S, Buchs NC, Addeo P, et al. Robot-assisted sleeve gastrectomy for super-morbidly obese patients. J Laparoendosc Adv Surg Tech A 2011;21:295-9. [Crossref] [PubMed]
- Diamantis T, Alexandrou A, Nikiteas N, et al. Initial experience with robotic sleeve gastrectomy for morbid obesity. Obes Surg 2011;21:1172-9. [Crossref] [PubMed]
- Vilallonga R, Fort JM, Gonzalez O, et al. The Initial Learning Curve for Robot-Assisted Sleeve Gastrectomy: A Surgeon's Experience While Introducing the Robotic Technology in a Bariatric Surgery Department. Minim Invasive Surg 2012;2012:347131 [Crossref] [PubMed]
- Vilallonga R, Fort JM, Caubet E, et al. Robotic sleeve gastrectomy versus laparoscopic sleeve gastrectomy: a comparative study with 200 patients. Obes Surg 2013;23:1501-7. [Crossref] [PubMed]
- Romero RJ, Kosanovic R, Rabaza JR, et al. Robotic sleeve gastrectomy: experience of 134 cases and comparison with a systematic review of the laparoscopic approach. Obes Surg 2013;23:1743-52. [Crossref] [PubMed]
- Sudan R, Bennett KM, Jacobs DO, et al. Multifactorial analysis of the learning curve for robot-assisted laparoscopic biliopancreatic diversion with duodenal switch. Ann Surg 2012;255:940-5. [Crossref] [PubMed]
- Sudan R, Podolsky E. Totally robot-assisted biliary pancreatic diversion with duodenal switch: single dock technique and technical outcomes. Surg Endosc 2015;29:55-60. [Crossref] [PubMed]
- Antanavicius G, Mohammed R, Van Houtte O. Total Robotic Biliopancreatic Diversion with Duodenal Switch Technique. Obes Surg 2017;27:1104-8. [Crossref] [PubMed]
- Antanavicius G, Katsichtis T, Alswealmeen W, et al. Three Hundred Four Robotically Assisted Biliopancreatic Diversion with Duodenal Switch Operations with Gradual Robotic Approach Implementation: Short-Term Outcomes, Complication Profile, and Lessons Learned. Obes Surg 2020;30:3961-7. [Crossref] [PubMed]
- Alqahtani A. Robotic gastric banding in children and adolescents: a comparative study. Surg Endosc 2011;25:3647-51. [Crossref] [PubMed]
- Moser F, Horgan S. Robotically assisted bariatric surgery. Am J Surg 2004;188:38S-44S. [Crossref] [PubMed]
- Mühlmann G, Klaus A, Kirchmayr W, et al. DaVinci robotic-assisted laparoscopic bariatric surgery: is it justified in a routine setting? Obes Surg 2003;13:848-54. [Crossref] [PubMed]
- Edelson PK, Dumon KR, Sonnad SS, et al. Robotic vs. conventional laparoscopic gastric banding: a comparison of 407 cases. Surg Endosc 2011;25:1402-8. [Crossref] [PubMed]
- Patel S, Szomstein S, Rosenthal RJ. Reasons and outcomes of reoperative bariatric surgery for failed and complicated procedures (excluding adjustable gastric banding). Obes Surg 2011;21:1209-19. [Crossref] [PubMed]
- Snyder B, Wilson T, Woodruff V, et al. Robotically assisted revision of bariatric surgeries is safe and effective to achieve further weight loss. World J Surg 2013;37:2569-73. [Crossref] [PubMed]
- Buchs NC, Pugin F, Azagury DE, et al. Robotic revisional bariatric surgery: a comparative study with laparoscopic and open surgery. Int J Med Robot 2014;10:213-7. [Crossref] [PubMed]
- Bindal V, Gonzalez-Heredia R, Elli EF. Outcomes of Robot-Assisted Roux-en-Y Gastric Bypass as a Reoperative Bariatric Procedure. Obes Surg 2015;25:1810-5. [Crossref] [PubMed]
- Esposito C, El Ghoneimi A, Yamataka A, et al. Work-related upper limb musculoskeletal disorders in paediatric laparoscopic surgery. A multicenter survey. J Pediatr Surg 2013;48:1750-6. [Crossref] [PubMed]
- Curet MJ, Curet M, Solomon H, et al. Comparison of hospital charges between robotic, laparoscopic stapled, and laparoscopic handsewn Roux-en-Y gastric bypass. J Robot Surg 2009;3:75-8. [Crossref] [PubMed]
Cite this article as: Bindal V, Sethi D, Pandey D. Robotic primary bariatric surgery. Dig Med Res 2021;4:56.