
Occult hepatitis B virus infection and the risk of hepatocellular carcinoma: a systematic review and meta-analysis
Introduction
Hepatocellular carcinoma (HCC) is the 6th most common cancer and 4th most common cancer-related mortality globally (1-3). As HCC can be treated by surgical resection or liver transplantation if discovered early, prompt detection of early HCC is important. In Asian countries where hepatitis B virus (HBV) infection is endemic, there is extremely high incidence of HCC (4,5). The primary diagnostic approach for HBV infection is detecting the presence of serum hepatitis B virus surface antigen (s.HBsAg). Hence, regular HCC surveillance of patients with positive s.HBsAg is recommended. In some cases, currently available serum assays do not detect HBsAg, even though the HBV DNA is present in serum or liver tissue in low quantities. The latter is termed occult hepatitis B virus infection (OBI). The loss of HBsAg in the OBI population impedes the detection process and its clinical significance is controversial. No standard guidelines have been established for the management, in particular HCC surveillance, of patients following their sero-clearance of HBsAg, albeit low grade or intermittent presence of HBV DNA cannot be excluded in these patients (6,7).
At the 2008 Taormina expert meeting on OBI, OBI was defined as presence of HBV DNA in the hepatocytes in s.HBsAg negative individuals with or without detectable viral DNA in the serum. The intermittent viral load in the serum should be less than 200 IU/mL (8). The persistence of HBV DNA in the host even after the infection is resolved has long been suspected and becomes evident when immunocompromised individuals who had received HBsAg negative blood go on to develop HBV infection (9-11).
OBI has been studied extensively for its clinical implications. First of all, from a public health point of view, OBI poses a potential risk for transmission though blood transfusion and liver transplantation, and in hemodialysis setting. There is evidence to support the proposition that, after transmission, the virus retains its ability to be reactivated, if the new host is immunocompromised (9,11). This makes OBI screening before transfusion or transplantation procedures a necessity in areas where HBV is endemic (10). In addition to the transmission, HBV persistence and its ability to integrate into host genome have sparked much interest in its potential role in the development of cancer (12,13).
The impact of OBI in the development of cancer in immunocompetent individuals has remained controversial. Since the early ‘90 s, numerous studies have demonstrated a high prevalence of HBV persistence in HBsAg negative individuals with HCC (14-16).
The gold standard for defining OBI is liver biopsy, which may not have been readily available for all studies and for all patients. The sample size of most of the studies tend to be small and heterogeneous. As theirs. HBsAg are negative, it is challenging to identify and follow this group who had asymptomatic or subclinical HBV infection that has since resolved, without resorting to expensive nucleic acid testing.
In view of the high mortality and morbidity of HCC, it is important to identify and risk-stratify the OBI population. This systematic review aims to reconcile the discrepancies of these studies based on standardized inclusion criteria to evaluate the association of occult hepatitis HBV infection and development of HCC, and provide information for the clinical management of this population.
We present the following article in accordance with the PRISMA reporting checklist (available at https://dx.doi.org/10.21037/dmr-21-50).
Methods
Literature search and eligibility criteria
Two independent reviewers, Weng and Kumar, performed literature searches in several databases (MEDLINE, Scopus, and ScienceDirect) for articles published up to December 2020, using key words such as “Occult hepatitis B infection, Hepatocellular carcinoma” and “Hepatitis B core positive, Hepatocellular carcinoma”. In addition, relevant references of included studies were used to screen for articles.
The eligibility criteria included: (I) nested PCR used to detect HBV DNA in either serum or liver tissues, (II) sufficient information to conduct either proportion analysis or odds ratio estimates, and (III) no other concomitant liver diseases (HIV, HCV). (IV) Only patients who have negative hepatitis B surface antigen included. We excluded studies lacking PCR detection method, studies with subjects who contained other concomitant liver diseases (HIV, HCV), studies not published in English and studies with overlapping data.
Data extraction
Two independent reviewers, Weng and Kumar, extracted relevant information from the selected studies. The data retrieved included: the study design, year and location of the study, sample size, relevant control and case numbers, duration of the study, number of occult hepatitis HBV infected patients who developed HCC, number of occult hepatitis HBV infected control subjects who did not have HCC, methods for HBV DNA detection with detection sensitivity, and potential confounding factors or bias.
Quality assessment
The quality of the studies is assessed based on study designs, sample size, presence of control or unexposed subjects, and detection methods. The detection methods are evaluated according to the number of primers used to target regions of HBV genome, presence of negative controls, and sensitivity of detection methods. Scores range from 1–8, with 6 to 8 indicating a high-quality study (Table 1) (17-20).
Table 1
| Quality parameters | Scores | ||
|---|---|---|---|
| 2 | 1 | 0 | |
| Study design | Cohort study or case control study | Case series study | – |
| Sample size | >150 | 51–150 | <50 |
| Detection methods | |||
| (I) Definition of OBI (number of positive primers targeting different regions of HBV genome) | – | Two or more primers from different areas of the HBV genome | One primer from one area of HBV genome |
| (II) Presence of negative control | – | Yes | No |
| (III) Detection lower limit | – | <50 copies/mL | >50 copies/mL |
| Comparing with control subjects or unexposed subjects | – | Yes | No |
Low quality studies, 0–2; medium quality studies, 3–5; high quality studies, 6–8 scores. OBI, occult hepatitis B virus infection; HBV, hepatitis B virus.
Statistical analysis
The association between OBI and HCC was evaluated using proportion analysis to compare the proportion of occult hepatitis HBV infected individuals in the HCC population and non-HCC population. The software used for the analysis was Review Manager 5.4.1. The heterogeneity of each study was assessed using I2 statistic, which measured diversity across the studies due to heterogeneity rather than chance (21), with I2>50% indicating significant heterogeneity across the studies. A random effect model was used irrespective of the substantial heterogeneity. The funnel plot of proportion analysis was evaluated for symmetry which indicated whether publication bias existed. Studies with high quality scores (6-8) were stratified to calculate the odds ratio for HCC in occult hepatitis HBV infected individuals. Occult hepatitis HBV infection was also compared with regard to gender and age outcomes. Finally, occult hepatitis HBV infection associated HCC was compared based on serological marker of HBV core and HBV surface.
Results
Selection and characteristics of included studies
A total of 1,612 studies were identified in the databases. After 683 duplicate studies to the same studies were excluded, 929 studies were screened on the basis of their titles and abstracts. Eight hundred and eighty studies were further excluded because they were: (I) not relevant to occult hepatitis B infection; (II) review studies; (III) studies which included patients with HIV/HCV; (IV) case-report studies; and (V) studies which could have included potentially immunocompromised patients or subjects with risk of other concomitant hepatitis virus infections, such as subjects on dialysis, intravenous drug users or patients undergoing transplantation. Fifty-nine articles underwent full-text review for the final evaluation, together with two additional references from other meta-analysis studies. In the end, two prospective studies, four case series and four retrospective studies were selected based on the eligibility criteria (Figure 1).
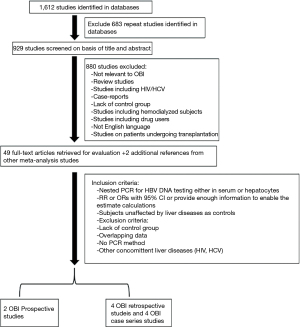
The four retrospective studies contained non-HCC control group that enabled odds ratio estimates. All the studies provided sufficient data to conduct proportion analyses. Five studies were conducted in Japan, two in Italy, and one each in Germany, Taiwan and China (Table 2) (14,22-30).
Table 2
| Study and year | Country/Region | Design | Age | Sex | Eligible number of participants | Quality scores |
|---|---|---|---|---|---|---|
| Knoll |
Germany | Prospective | Yes | Yes | 42 | 2 |
| Ikeda |
Japan | Prospective | Yes | Yes | 82 | 6 |
| Ohba |
Japan | Retrospective | Yes | Yes | 14 | 3 |
| Muto |
Japan | Retrospective | Yes | Yes | 36 | 3 |
| Kusakable |
Japan | Retrospective | Yes | Yes | 51 | 3 |
| Coppola |
Italy | Retrospective | Yes | Yes | 68 | 4 |
| Yotsuyanagi |
Japan | Retrospective | Yes | Yes | 84 | 6 |
| Chen |
Taiwan | Retrospective | Yes | Yes | 522 | 7 |
| Pollicino |
Italy | Retrospective | Yes | Yes | 299 | 7 |
| Fang |
China | Retrospective | Yes | Yes | 494 | 7 |
The association between occult hepatitis B viral infection and HCC
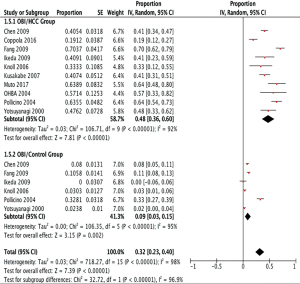
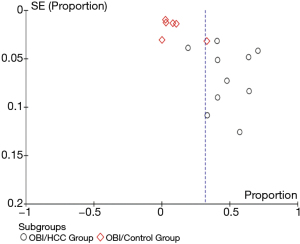
Pooled unadjusted proportion analysis was conducted to evaluate the proportion of OBI according to the HCC profile (Figure 2). The pooled overall proportion of OBI was 0.48 (95% CI: 0.36, 0.60) in a total of 706 patients with HCC, which was much higher than the pooled overall proportion of OBI, 0.09 (95% CI: 0.03, 0.15), in a total of 986 patients without HCC (P<0.00001). This indicates that OBI is strongly associated with HCC. Significant heterogeneity among the included studies was found using I2 measurement (I2=96.9%). A sensitivity analysis was performed by excluding the study with the largest sample size and several low-quality studies evaluated by the quality assessment (score of 0–2). The exclusion of the studies did not alter the overall trend of the conclusion but decreased slightly the difference between the groups being compared. The asymmetry of the funnel plot for the proportion analysis indicated that possible publication bias exists (Figure 3).
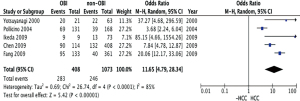
The studies were stratified by quality assessment and the odds ratio for HCC in OBI population was 11.65 (95% CI: 4.79, 28.34; P<0.00001) from high quality studies with scores of 6–8 (Figure 4). This further supports the proposition that OBI increases the risk of HCC. With stratification, heterogeneity among the studies decreased (I2=85%).
The roles of gender and age in the OBI
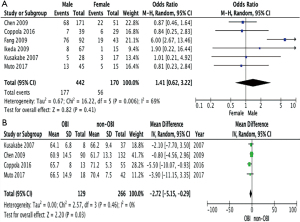
The incidence of OBI in men was compared with the incidence of OBI in women and the pooled unadjusted odds ratio was 1.41 (95% CI: 0.62, 3.22; P=0.41), that is, OBI in men and women were similarly likely to be associated with HCC. When the ages of individuals in the OBI and non-OBI groups were compared, the mean difference was −2.72 (95% CI: 5.15, −0.29; P=0.03), indicating that occult HBV associated HCC tends to occur in a younger population (Figure 5).
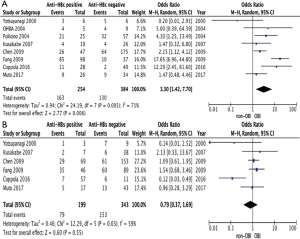
The associations between OBI related HCC and anti-Hepatitis B core antibody and anti-Hepatitis B surface antibody
In clinical settings, anti-HBc Ab indicates past exposure to HBV infection. Hence, it is reasonable to assume that all OBI patients should have positive anti-HBc Ab. This is not the case, however, when data from included papers were collected (Table 3). Some of HCC patients with OBI were not seropositive for either anti-HBc Ab or anti-HBs Ab. To investigate the association between OBI-related HCC and the serology markers, odd ratio estimates were calculated. The pooled unadjusted odd ratios for the included retrospective studies comparing the incidence of OBI- related HCC regarding the status of anti-HBc Ab (+/−) and anti-HBs Ab (+/−) were 3.30 (95% CI: 1.42, 7.70; P=0.006) and 0.79 (95% CI: 0.37, 1.69; P=0.55) respectively. This suggests that both HBV serology markers (anti-HBc Ab and anti-HBs Ab) are positively associated with the risk of OBI-related HCC. Furthermore, there is a higher degree of association with anti-HBc Ab than anti-HBs Ab (Figure 6A,B).
Table 3
| Serological markers | OBI | Non-OBI |
|---|---|---|
| Anti-HBc (+/− anti-HBs) | 153/293 | 91/345 |
| Anti-HBs (+/− anti-HBc) | 79/232 | 120/310 |
OBI, occult hepatitis B virus infection; HBV, hepatitis B virus.
Discussion
In this study, the quality of the selected studies was assessed, in part, by the sensitivity of the detection methods. Using proportion analysis, where HCC population was matched with healthy controls without HCC, the meta-analysis of the pooled data from the ten selected papers revealed a strong association between occult hepatitis B infection and HCC (P<0.00001). A sensitivity analysis was also performed to study the impact of different studies on the conclusion. After excluding the largest study and several small studies, the conclusion was consistent. With quality stratification, high quality studies were pooled to compare occult HBV infected group with negative hepatitis B DNA exposure group, and the calculated odds ratio of HCC in OBI is 11.65 (95% CI: 4.79, 28.34; P<0.00001), further confirming that OBI is associated with HCC.
The significant heterogeneity (I2 value >50%) among the selected studies is worth mentioning. The reduced heterogeneity in quality stratification (I2=97 to I2=85%) indicated that the variances arose largely from the study design, risk of bias, study methodology and lab detection methods. In addition, other risk factors that were difficult to monitor or quantify in the studies, such as heavy alcohol intake, genetic predisposition, or occupational risk, could have contributed to the heterogeneity of the population and confounded the results. Moreover, the risk of HCC is expected to vary within the pure OBI group, even in the absence of confounding factors. Individuals who became OBI only after decades of HBsAg-positive chronic hepatitis B would have sustained far more liver damage than individuals who have OBI at, or near, the start of HBV infection, with decades of relatively quiescent clinical state that has been associated with low grade, possibly only intermittent, viraemia (31-33). In addition, members of different communities or risk groups (e.g., patients with transplantations), with differing lifestyles and subjected to variable epidemiologic factors, also contributed to the heterogenous outcome of this population.
Several studies indicated that individuals with OBI, who had prior liver injuries or concomitant liver diseases, developed liver adverse events through either direct or indirect mechanisms. The suggested indirect mechanism involves the mediated immune response that was provoked by the low but persistent viraemia, giving rise to progressive liver damage and eventually liver cancer in the OBI population. In clinical studies (34), intermittently transient reactivation of viral replication has been observed promoting the response of host immune system such as cytokines or anti-HBV specific T lymphocyte in OBI patients (35-38). The resulting inflammation induces liver damage and promote cellular turnover that eventually leads to cirrhosis and HCC.
Looking at the characteristics of gender and age in HCC patients, our study demonstrated that, there was no significant difference in the risk of OBI associated HCC between men and women. However, there was an association of OBI-related HCC with a younger population (between early to mid-60s), compared with non-OBI related HCC, which was associated with an older population (from late 60s to early 70s). The start of seroconversion of HBsAg were reported to occur near the age of 40, with average duration of the seroconversion course over 6 years (39-41). Based on the mean age of OBI-related HCC in our study, there should be sufficient time for OBI associated mutations to give rise to OBI—specific carcinogenesis, thus possible OBI—specific mechanism as cause of HCC, post seroclearance of HBsAg.
Studies have been conducted to assess the association between HBV serological markers and occult HBV infection. The results have been controversial. Some studies suggest that OBI is more prevalent in patients who are both anti-HBc Ab and anti-HBs Ab positive but not either anti-HBc Ab or anti-HBs Ab positive only (20). The rationale for such an observation is that patients who recovered from acute hepatitis B tend to be positive for both anti-HBc Ab and anti-HBs Ab and as this patient population can have persistent HBV DNA in liver tissues, it is more likely to develop OBI. Other studies have linked anti-HBc Ab with occult HBV infection (42,43) and a higher risk of HCC. This should not be surprising as anti-HBc Ab is an indication of past HBV infection, which is a risk of HCC, as well as a predisposing factor of OBI. As anti-HBs Ab are thought to act as immune control, HBsAg-negative patients without anti-HBs Ab are thought to be more likely to develop HCC. To investigate these discrepancies, our analysis pooled the data from selected retrospective studies. The pooled unadjusted odd ratios comparing the incidence of OBI related HCC in relation to the status of anti-HBc Ab (+/−) and anti-HBs Ab (+/−) in HCC patients are 3.30 (95% CI: 1.42, 7.70; P=0.006) and 0.79 (95% CI: 0.37, 1,69; P=0.55), indicating both anti-HBc Ab and anti-HBs Ab are positively associated with OBI related HCC. This result is of practical clinical value. As OBI status is hard to confirm without a liver biopsy or an expensive nucleic acid test, anti-HBc Ab can be used to prompt investigation of OBI and surveillance of HCC. Furthermore, with a higher odds ratio estimate, anti-HBc Ab shows a stronger positive association with OBI related HCC than anti-HBs Ab. This supports previous studies that suggested the risk reduction of HCC with presence of anti-HBs Ab. More detailed investigations need to be conducted, as individuals who were both anti-HBc Ab and anti-HBs Ab positive were not isolated in selected studies. Hence the association between OBI-related HCC and lone seropositivity of anti-HBc Ab or anti-HBs Ab cannot be determined.
An asymmetric funnel plot was observed in this study, indicating possible publication bias. Causes for publication bias include: the suppression of negative results in smaller studies, “time-lag” publication or unpublished clinical studies, and the exclusion of high-quality studies which had been published in languages other than English. The studies’ heterogeneity, as mentioned above, could also have influenced the symmetry of the funnel plot.
There are some limitations in this meta-analysis. In addition to the heterogeneity of the studies and potential publication bias, the study populations were also geographically limited. Most of the studies were conducted in East Asia or Italy where HBV infection are of higher endemicity. Studies published in languages other than English were not considered, thus potentially omitting high quality studies in non-English publications. As viral loads and viral genotypes were only described in a few studies, there was not enough data to investigate whether viral load or a specific HBV genotype played a role in OBI-induced HCC.
In conclusion, our study revealed a strong association between occult HBV infection and the development of HCC. The OBI-related HCC was associated with a slightly younger population, of age early to mid-60 years. Anti-HBc Ab, which is associated with the risk of OBI-related HCC, can be used to identify at-risk patients for HCC screening, where appropriate. While this study does not specifically address the patient population who were treated with antiviral agents, this finding should caution us to review the management of patients who have lost HBsAg following anti-viral agents. Future studies should investigate the association between OBI and progression of liver disease and cirrhosis. In addition, studies to investigate the specific genetic and molecular mechanisms that drives the development of HCC in patients with OBI are important in the development of new therapeutic options.
Acknowledgments
Funding: This work was supported by DukeNUS Medical Student Fellowship from SingHealth Duke-NUS Joint Office of Academic Medicine.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/dmr-21-50
Peer Review File: Available at https://dx.doi.org/10.21037/dmr-21-50
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/dmr-21-50). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- Gadiparthi C, Yoo ER, Are VS, et al. Hepatocellular carcinoma is leading in cancer-related disease burden among hospitalized baby boomers. Ann Hepatol 2019;18:679-84. [Crossref] [PubMed]
- Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589-604. [Crossref] [PubMed]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-76. [Crossref] [PubMed]
- MacLachlan JH, Cowie BC. Hepatitis B virus epidemiology. Cold Spring Harb Perspect Med 2015;5:a021410 [Crossref] [PubMed]
- Roman S. Occult Hepatitis B and Other Unexplored Risk Factors for Hepatocellular Carcinoma in Latin America. Ann Hepatol 2018;17:541-3. [Crossref] [PubMed]
- Mak LY, Wong DK, Pollicino T, et al. Occult hepatitis B infection and hepatocellular carcinoma: Epidemiology, virology, hepatocarcinogenesis and clinical significance. J Hepatol 2020;73:952-64. [Crossref] [PubMed]
- Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol 2008;49:652-7. [Crossref] [PubMed]
- Marzano A, Angelucci E, Andreone P, et al. Prophylaxis and treatment of hepatitis B in immunocompromised patients. Dig Liver Dis 2007;39:397-408. [Crossref] [PubMed]
- Seo DH, Whang DH, Song EY, et al. Occult hepatitis B virus infection and blood transfusion. World J Hepatol 2015;7:600-6. [Crossref] [PubMed]
- Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis 2002;2:479-86. [Crossref] [PubMed]
- Gozuacik D, Murakami Y, Saigo K, et al. Identification of human cancer-related genes by naturally occurring Hepatitis B Virus DNA tagging. Oncogene 2001;20:6233-40. [Crossref] [PubMed]
- Tan YJ. Hepatitis B virus infection and the risk of hepatocellular carcinoma. World J Gastroenterol 2011;17:4853-7. [Crossref] [PubMed]
- Pollicino T, Squadrito G, Cerenzia G, et al. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology 2004;126:102-10. [Crossref] [PubMed]
- Ruiz J, Sangro B, Cuende JI, et al. Hepatitis B and C viral infections in patients with hepatocellular carcinoma. Hepatology 1992;16:637-41. [Crossref] [PubMed]
- Wong DK, Huang FY, Lai CL, et al. Occult hepatitis B infection and HBV replicative activity in patients with cryptogenic cause of hepatocellular carcinoma. Hepatology 2011;54:829-36. [Crossref] [PubMed]
- Covolo L, Pollicino T, Raimondo G, et al. Occult hepatitis B virus and the risk for chronic liver disease: a meta-analysis. Dig Liver Dis 2013;45:238-44. [Crossref] [PubMed]
- Liu S, Zhang H, Gu C, et al. Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: a meta-analysis. J Natl Cancer Inst 2009;101:1066-82. [Crossref] [PubMed]
- Pisaturo M, Onorato L, Russo A, et al. An estimation of the prevalence of occult HBV infection in Western Europe and in Northern America: A meta-analysis. J Viral Hepat 2020;27:415-27. [Crossref] [PubMed]
- Shi Y, Wu YH, Wu W, et al. Association between occult hepatitis B infection and the risk of hepatocellular carcinoma: a meta-analysis. Liver Int 2012;32:231-40. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Knöll A, Hartmann A, Hamoshi H, et al. Serological pattern "anti-HBc alone": characterization of 552 individuals and clinical significance. World J Gastroenterol 2006;12:1255-60. [Crossref] [PubMed]
- Ikeda K, Kobayashi M, Someya T, et al. Occult hepatitis B virus infection increases hepatocellular carcinogenesis by eight times in patients with non-B, non-C liver cirrhosis: a cohort study. J Viral Hepat 2009;16:437-43. [Crossref] [PubMed]
- Ohba K, Kubo S, Tamori A, et al. Previous or occult hepatitis B virus infection in hepatitis B surface antigen-negative and anti-hepatitis C-negative patients with hepatocellular carcinoma. Surg Today 2004;34:842-8. [Crossref] [PubMed]
- Muto J, Sugiyama M, Shirabe K, et al. Frequency and Characteristics of Occult Hepatitis B Infection Among Hepatocellular Carcinoma Patients in Japan. Ann Hepatol 2018;17:596-603. [Crossref] [PubMed]
- Kusakabe A, Tanaka Y, Orito E, et al. A weak association between occult HBV infection and non-B non-C hepatocellular carcinoma in Japan. J Gastroenterol 2007;42:298-305. [Crossref] [PubMed]
- Coppola N, Onorato L, Iodice V, et al. Occult HBV infection in HCC and cirrhotic tissue of HBsAg-negative patients: a virological and clinical study. Oncotarget 2016;7:62706-14. [Crossref] [PubMed]
- Yotsuyanagi H, Shintani Y, Moriya K, et al. Virologic analysis of non-B, non-C hepatocellular carcinoma in Japan: frequent involvement of hepatitis B virus. J Infect Dis 2000;181:1920-8. [Crossref] [PubMed]
- Chen CH, Changchien CS, Lee CM, et al. A study on sequence variations in pre-S/surface, X and enhancer II/core promoter/precore regions of occult hepatitis B virus in non-B, non-C hepatocellular carcinoma patients in Taiwan. Int J Cancer 2009;125:621-9. [Crossref] [PubMed]
- Fang Y, Shang QL, Liu JY, et al. Prevalence of occult hepatitis B virus infection among hepatopathy patients and healthy people in China. J Infect 2009;58:383-8. [Crossref] [PubMed]
- Ahn SH, Park YN, Park JY, et al. Long-term clinical and histological outcomes in patients with spontaneous hepatitis B surface antigen seroclearance. J Hepatol 2005;42:188-94. [Crossref] [PubMed]
- Malagnino V, Fofana DB, Lacombe K, et al. Occult Hepatitis B Virus Infection: An Old Entity With Novel Clinical Involvements. Open Forum Infect Dis 2018;5:ofy227 [Crossref] [PubMed]
- Minuk GY, Kowalec K, Caouette S, et al. The prevalence and long term outcome of occult hepatitis B virus infections in community based populations. J Med Virol 2012;84:1369-75. [Crossref] [PubMed]
- Chemin I, Guillaud O, Queyron PC, et al. Close monitoring of serum HBV DNA levels and liver enzymes levels is most useful in the management of patients with occult HBV infection. J Hepatol 2009;51:824-5. [Crossref] [PubMed]
- Germanidis G, Hytiroglou P, Zakalka M, et al. Reactivation of occult hepatitis B virus infection, following treatment of refractory rheumatoid arthritis with abatacept. J Hepatol 2012;56:1420-1. [Crossref] [PubMed]
- Martin CM, Welge JA, Shire NJ, et al. Cytokine expression during chronic versus occult hepatitis B virus infection in HIV co-infected individuals. Cytokine 2009;47:194-8. [Crossref] [PubMed]
- Penna A, Artini M, Cavalli A, et al. Long-lasting memory T cell responses following self-limited acute hepatitis B. J Clin Invest 1996;98:1185-94. [Crossref] [PubMed]
- Rehermann B, Ferrari C, Pasquinelli C, et al. The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med 1996;2:1104-8. [Crossref] [PubMed]
- Kim GA, Lee HC, Kim MJ, et al. Incidence of hepatocellular carcinoma after HBsAg seroclearance in chronic hepatitis B patients: a need for surveillance. J Hepatol 2015;62:1092-9. [Crossref] [PubMed]
- Park YM, Lee SG. Clinical features of HBsAg seroclearance in hepatitis B virus carriers in South Korea: A retrospective longitudinal study. World J Gastroenterol 2016;22:9836-43. [Crossref] [PubMed]
- Simonetti J, Bulkow L, McMahon BJ, et al. Clearance of hepatitis B surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis B virus. Hepatology 2010;51:1531-7. [Crossref] [PubMed]
- Fattovich G, Olivari N, Pasino M, et al. Long-term outcome of chronic hepatitis B in Caucasian patients: mortality after 25 years. Gut 2008;57:84-90. [Crossref] [PubMed]
- Sagnelli E, Coppola N, Scolastico C, et al. HCV genotype and "silent" HBV coinfection: two main risk factors for a more severe liver disease. J Med Virol 2001;64:350-5. [Crossref] [PubMed]
Cite this article as: Weng C, Kumar R, Sultana R, Chow WC. Occult hepatitis B virus infection and the risk of hepatocellular carcinoma: a systematic review and meta-analysis. Dig Med Res 2021;4:46.


