
Targeted therapy in esophageal cancer
Introduction
Esophageal cancer consists of two distinct histological types, esophageal squamous cell-carcinoma (ESCC) and esophageal adenocarcinoma (EAC). While squamous cell carcinoma is mostly found in the upper and middle parts of the esophagus, adenocarcinoma starts with the glandular cells and occurs usually in the lower part of the esophagus (1). Esophageal cancer remains the eighth most common cancer worldwide (2). The variation in incidence of esophageal cancer is geographical with ESCC continuing to be the major type in Asia (2), and EAC the dominant type in the United States and the western world (1,3-5). EAC is one of the most aggressive human cancers, and the only major cancer in the United States with increasing incidence (1,6,7). In addition, EAC can occur at the gastric junction, known as esophagogastric junction adenocarcinoma (GEJAC) (8). Gastroesophageal reflux disease (GERD) and obesity are the main known risk factors for EAC and obesity can cause or worsen GERD (2,9,10). In addition, GERD can cause Barrett’s esophagus (BE), the recognized precursor lesion consisting of columnar metaplasia of the lower esophagus. BE remains the strongest known risk factor for the development of EAC (11-13). It is believed that Barrett’s metaplasia progresses through low to high grade dysplasia before developing into adenocarcinoma (14-16). The main risk factors of ESCC are tobacco smoking, alcohol consumption, hot beverage drinking, and poor nutrition. Unfortunately, overall 5-year survival rates for all stages of esophageal cancer are still below 20 percent. Despite significant progress in multimodality treatment options, the overall prognosis remains poor due to high resistance to chemotherapy (17,18). In addition, most esophageal cancers are already unresectable by the time of diagnosis (19). Although esophageal cancer initially responds well to systemic therapy, most patients recur and eventually die from their disease (20). Therefore, new treatment options are needed. With the identification of new biomarkers for esophageal cancers (21), targeted therapies are gaining interest (22). In esophageal cancer, several potentially targetable pathways have been identified (23). These targets include the human epidermal growth factor receptor 2 (HER2, Neu, ErbB2) (23), the epidermal growth factor receptor (EGFR, Her1, ErbB1) (24), the vascular endothelial growth factor (VEGF) (25), the mesenchymal-epithelial transition (MET) factor (26), and the programmed death ligand 1 (PD-L1) (27). Drugs targeting these proteins have been developed, brought into preclinical studies and clinical trials, and have shown clinical benefits (Table 1).
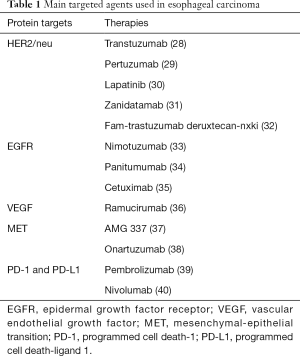
Full table
HER2
The human epidermal growth factor receptor 2 (HER2, Neu, ErbB2) emerged as a major target (41). Abnormal frequencies of HER2/neu have been identified in cancers other than breast cancer, making it a good target for therapies (42). HER is associated with cancer progression and a subset (0-83%) of esophageal cancer has been shown to overexpress HER2 (43-45). Thus blocking HER2 with a drug could increase esophageal cancer tumor regression and therapeutic efficacy in HER2 expressing esophageal cancer (46).
Trastuzumab
Trastuzumab (Herceptin), is a monoclonal antibody to HER2, and was the first FDA approved HER2 targeted agent for gastroesophageal (GE) cancer (47). Trastuzumab was initially used to treat HER-2 positive breast cancer (48). Trastuzumab was shown to inhibit the growth of esophageal cancers with HER2 expression (49). When added to chemotherapy for HER2-positive advanced gastric and gastro-esophageal junction cancers, there was a significant increase in median overall survival (OS) with a hazard ratio (HR) of 0.74; and a 95% confidence interval (CI) of 0.60–0.91; P=0.0046 (28).
Pertuzumab
Pertuzumab, a humanized monoclonal antibody that also targets the HER2 receptor, similar to trastuzumab. However, it binds to a different epitope of the HER2 receptor. Pertuzumab, in combination with trastuzumab and docetaxel demonstrated improved survival in patients with HER2-positive breast cancer (50). However, in the phase 3 JACOB trial, the addition of pertuzumab to trastuzumab plus chemotherapy showed no significant improvement in survival of patients with HER-2 positive gastro-esophageal cancer (29). The median overall survival (OS) was 17.5 months (95% CI: 16.2–19.3) in the treatment group with pertuzumab, and 14.2 months (95% CI: 12.9–15.5) in the control group. While a statistically significant difference in overall survival was not reached between the two groups with an HR of 0.84 (95% CI: 0.71–1.00); P=0.057, there was a clinical survival benefit of more than 3 months. Furthermore, in a Chinese subpopulation analysis of the JACOB trial, the median overall survival was 18.7 months in the treatment group as opposed to 16.1 months in the control group (HR 0.75; 95% CI: 0.49–1.14). The authors concluded that overall survival was numerically improved by addition pertuzumab to trastuzumab and chemotherapy (51). The results from the Japanese subgroup analysis of the JACOB trial suggested a similar effect of pertuzumab in Japanese patients (22 months in the pertuzumab group, versus 15.6 months in the placebo group) (52).
Zanidatamab (ZW25)
Zanidatamab is a Her-2 targeted biparatopic antibody that binds to two different domains of Her-2 at the same time (ECD4 and ECD2). In a recently reported phase 1 study, this agent showed antitumor activity with good tolerability in patients with HER2-expressing GE adenocarcinoma who had progression of disease after standard-of-care therapy, with an overall response rate (ORR) of 33% and a disease control rate of 61% as monotherapy, and an ORR of 54% and a disease control rate of 79% in combination with chemotherapy (31).
Fam-trastuzumab deruxtecan-nxki
Fam-trastuzumab deruxtecan-nxki is a HER2-directed antibody and a topoisomerase inhibitor drug conjugate. It was approved by the FDA in January 2012 for the treatment of patients with progressing locally advanced or metastatic Her-2 positive gastric or GE adenocarcinoma, who were treated with at least two prior regimens. In the DESTINY-Gastric01 trial (32), a response rate of 51% was observed in the trastuzumab deruxtecan group, compared to 14% in the control group. The median overall survival was 12.5 months in the trastuzumab deruxtecan group, and 8.4 months in the control chemotherapy group (HR for death 0.59; 95% CI: 0.39–0.88; P=0.01).
EGFR
EGFR (Her1, ErbB1) is a receptor tyrosine kinase that belongs to the ErbB family that includes three other members (ErbB2/HER2/Neu, ErbB3/HER3 and ErbB4/HER4). Activation of EGFR leads to phosphorylation of the receptor which then activates several downstream effectors, such as the RAS-RAF-MEK-ERK-MAPK and the PI3K-AKT-mTOR pathways (53). EGFR overexpression has been reported in esophageal cancer, and anti-EGFR agents are used in clinical targeting of this receptor (54,55). To date addition of an anti-EGFR agent to chemotherapy seems to convey no additional benefit for patients with esophageal cancer (55,56), but the data remains inconclusive.
Nimotuzumab
Nimotuzumab is a fully recombinant, humanized monoclonal antibody against EGFR. A phase 1 study is looking at the use of nimotuzumab together with neoadjuvant chemotherapy before surgery for advanced esophageal cancer (33). The addition of nimotuzumab in combination with chemotherapy showed a significant anticancer effect with tolerable toxicities in advanced esophageal cancer. A phase 2 long-term follow-up study looked at the use of nimotuzumab in combination with paclitaxel and cisplatin as first line treatment for esophageal cancer (57). This study concluded that patients with limited local lymph node metastasis survived longer (the median OS was 26.2±10.0 months, with a 95% CI: of 6.6–45.8) than patients with distant metastasis (the median OS was 11.5±3.7 months, with a 95% CI: of 4.2–18.8) with the nimotuzumab, paclitaxel, and cisplatin combination therapy.
Panitumumab
Panitumumab is a fully human monoclonal IgG antibody targeting the EGFR. A phase 3 trial of panitumumab together with cisplatin and 5-fluorouracil failed to show an improvement in patient survival (34). Another phase 3 trial with panitumumab showed that adding it to chemotherapy as a first line treatment was found to be ineffective in advanced GE cancer (58).
Cetuximab
Cetuximab is an FDA approved chimeric (mouse/human) monoclonal antibody that is used to treat patients with advanced metastatic colorectal cancer. It targets EGFR and inhibits its activation, preventing cancer progression. Several studies have demonstrated that cetuximab can be used to treat esophageal cancer, especially in combination with chemotherapies (59). In a phase Ib/II trial, the addition of cetuximab to preoperative chemoradiotherapy showed its feasibility and higher 5 years survival rates in patients with squamous histology than that of adenocarcinoma (58% versus 25%, P=0.011) (35). Another phase II trial however showed that although still promising, the drug was correlated with high toxicity (60). The Swiss Group of Clinical Cancer Research phase III trial (SAKK 75/08) showed that the addition of cetuximab to multimodal therapy significantly improved the loco-regional control, leading to a clinically, but not statistically significant improvement of overall survival [5.1 years (95% CI: 3.7 to not reached) versus 3.0 years (95% CI, 2.2–4.2), HR, 0.73; 95% CI: 0.52–1.01; P=0.055) (61).
Lapatinib—a dual EGFR and HER2 inhibitor
Lapatinib is a potent ATP-competitive inhibitor which is available in oral preparation that simultaneously inhibits both EGFR and HER2, and is used in a phase 2 clinical trial (30). This randomized phase 2 study investigated the addition of lapatinib to standard perioperative chemotherapy (30). Administration of lapatinib in combination with chemotherapy was feasible, and did not affect the operative management, but the toxic effects of the drug were higher (30).
VEGF
The VEGF is a signaling protein that is involved in tumor angiogenesis, involving several complex processes (62). VEGF (VEGF-A, VEGF-B, VEGF-C, VEGF-D and PIGF) is produced by multiple cell types including tumor cells, and binds with VEGF receptor (VEGFR1, VEGFR2, VEGFR3) to activate various signaling pathways related to tumor growth, among them the extracellular regulated protein kinases 1/2 (ERK1/2) and the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathways (62). Thus the VEGF/VEGFR signaling pathway seems to be a potential target for esophageal cancer therapy.
Ramucirumab
Ramucirumab is a VEGFR-2 antagonist fully humanized monoclonal antibody. When added to cisplatin and fluoropyrimidine in the first-line therapy for patients with metastatic gastric or junctional adenocarcinoma (RAINFALL Trial), it failed to demonstrate improvement in patient outcome (36). However, in the RAINBOW trial (63), a significant increase in overall survival was present when patients were given ramucirumab plus paclitaxel versus paclitaxel alone, median OS of 9.6 months [95% CI: 8.5–10.8] vs. 7.4 months [95% CI: 6.3–8.4], HR 0.807 [95% CI: 0.678–0.962]; P=0.017. Ramucirumab (CYRAMZA) is now approved for the treatment of patients with advanced or metastatic gastric or GE junction cancer.
MET
The tyrosine protein kinase MET is a receptor for the hepatocyte growth factor (HGF). HGF-MET ligand-receptor interaction leads to tumor cell growth, invasion and metastasis (64). Several studies have demonstrated that MET is overexpressed in a subset of esophageal cancer, and that this overexpression is related to poor prognosis (65). In addition, MET-HER2 co-overexpression is also frequent in esophageal cancer patients (66), and they may be resistant to lapatinib, a dual EGFR and HER2 inhibitor therapy (26,67,68). Thus MET-HER2 targeting could be a new treatment approach for esophageal cancer that have overexpression or activation of MET and HER2 (26).
AMG337
AMG337 is a small molecule selective inhibitor of the MET receptor that has been shown to inhibit c-MET/HGF binding (69). Phase I and phase II clinical trials have shown that AMG337 is associated with antitumor activity in MET-amplified gastric, GE junction, and esophageal cancer patients (37).
Onartuzumab
Onartuzumab is a recombinant, fully humanized anti-MET monoclonal antibody that can inhibit the binding of MET to HGF, thereby restricting cellular signaling via the MET pathway. The results of phase II and phase III clinical trials of onartuzumab, in combination with FOLFOX chemotherapy in GE adenocarcinoma could not show improved progressive-free-survival in MET-positive patients (38,70). Furthermore, phase III trials have failed to show a survival benefit (68/69) and did not improve outcome in patients (38).
Although clinical trials of other inhibitory anti-MET antibodies like rilotumumab (AMG-102) (71) failed to demonstrate any survival benefits in esophageal cancer patients, further research is required to better elucidate which patient populations may potentially benefit from these therapies.
PD-1 and PD-L1
The PD-1 (programmed cell death-1) receptor is expressed on the surface of activated T cells, and its ligand, PD-L1 (is expressed on the surface of dendritic cells or macrophages. Cancer cells exhibit immune escape by the expression of PD-L1 when PD-L1 on cancer cells bind to PD-1 on immune cells. Therefore, PD-1 and PD-L1 targeted therapy blocking this interaction is considered an effective therapy for a multitude of different tumors (72). PD-L1 protein expression in gastric or GEJ adenocarcinoma is determined by using the combined positive score (CPS). This score is calculated by the number of PD-L1 stained cells (tumor cells, lymphocytes, and macrophages), divided by the number of viable tumor cells, and multiplied by 100 (73).
Pembrolizumab
Pembrolizumab is a humanized monoclonal IgG4 kappa antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thus preventing PD-L1 on cancer cells from binding to the PD-1 receptor on T cells, and therefore enabling these T cells to kill cancer cells. Pembrolizumab is approved by the FDA for the treatment of PD-L1 positive tumors in patients with advanced GE junction adenocarcinoma, who received at least two prior lines of chemotherapy (39). The approval was granted based on the demonstration of a durable ORR in the multi-centerKEYNOTE-059 trial (74). The FDA also granted approval for pembrolizumab as a second line option for PD-L1 positive ESCC, based on the KEYNOTE-180 phase II and KEYNOTE-181 phase III trials (75,76). The KEYNOTE-590 trial demonstrated that pembrolizumab and chemotherapy significantly improved overall survival, progression-free survival, and objective response rates as compared to chemotherapy alone in patients with locally advanced, unresectable or metastatic esophageal cancer, with a median overall survival of 12.4 months (95% CI: 10.5–14.0) vs. 9.8 months (77).
Nivolumab
Nivolumab is a PD-1 blocking antibody that has been approved for a variety of metastatic or locally advanced tumors, including esophageal squamous cell carcinoma. In the phase III CheckMate 649 trial, nivolumab, in combination with chemotherapy, showed superior overall survival and progression-free survival as first-line treatment in patients with unresectable advanced, or metastatic GC/GEJC/EAC with a median overall survival of 14.4 months in the nivolumab plus chemotherapy arm, vs. 11.1 months for the chemotherapy arm in the PD-L1 CPS ≥5 population (HR =0.71; P<0.0001). In addition, there was also a statistically significant OS benefit in patients with PD-L1 CPS ≥1, as well as the all-randomized population (40). Moreover, Nivolumab, administered as adjuvant treatment following neoadjuvant chemoradiation and complete surgical resection in patients with esophageal or GE junction cancer, demonstrated statistically significant improvement in disease-free survival (DFS) in the phase 3 CheckMate-577 trial (78) with a doubling of DFS (22.4 vs. 11.0 months).
Combination therapies and new emerging targets
Several clinical trials with combinations of the above described targets are ongoing, such as the combination of PD-L1 or PD-1 directed therapy with Her-2 based therapy, as in the MAHAGONY trial with margetuximab plus checkpoint inhibitors (79), or the combination of check point inhibition with trastuzumab and chemotherapy (80,81).
One of the new emerging targets is claudin 18.2. This is a tight junction protein that is expressed in the gastric epithelia, and is retained in malignancy (82). Zolbetuximab, a chimeric IgG1 monoclonal antibody against claudin 18.2, showed promising results in a phase II trial (83). The phase III GLOW trial will investigate Zolbetuximab in combination with CAPOX chemotherapy in gastric and GE junction cancer (84). The Spothlight trial will use the same antibody in combination with mFOLFOX chemotherapy (85).
Another emerging target is the Fibroblast growth factor receptor 2 (FGFR2). FGFR2 overexpression has been shown to be associated with tumor cell proliferation, cell cycle progression, and anti-apoptosis in adenocarcinoma of the GE junction (86). The FiGhTeR trial is a phase II study investigating the FGFR inhibitor pemigatinib as second-line therapy in metastatic gastroesophageal adenocarcinoma or gastric cancer patients who were progressing under trastuzumab-containing therapies (87).
Conclusions
EAC which resembles gastric/GEJ adenocarcinoma evolved into leading type of esophageal cancer in the US, and is one of the fastest growing cancers in the Western World. In contrary, in Asia, esophageal squamous cells carcinoma (ESCC) is far more common than EAC. Despite significant progress in multimodality treatment options, the overall prognosis remains poor, and most patients survive less than one year on average. Therefore, new therapeutic approaches using targeted drugs with good efficacy and limited side effect are urgently needed. HER-2 (trastuzumab), VEGFR (ramucirumab) and PD-L1 (pembrolizumab) targeted therapies have demonstrated improved survival and prognosis in advanced EAC and ESCC. Clinical trials are currently underway to explore the benefits of combination treatments, including cytotoxic and targeted agents with different mechanisms of actions to improve the survival of patients with esophageal cancer.
Acknowledgments
Funding: Indiana University School of Medicine and Goshen Center for Cancer Care.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Niranjan Awasthi and Changhua Zhang) for the series “Targeted Therapy in Gastrointestinal Cancers” published in Digestive Medicine Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-21-16). The series “Targeted Therapy in Gastrointestinal Cancers” was commissioned by the editorial office without any funding or sponsorship. Dr. von Holzen reports other from Novartis, other from Roche, outside the submitted work. All authors have no other conflicts of interest to declare.
Ethical Statement: All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014;371:2499-509. [Crossref] [PubMed]
- Zhang HZ, Jin GF, Shen HB. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer 2012;31:281-6. [Crossref] [PubMed]
- Chai J, Jamal MM. Esophageal malignancy: a growing concern. World J Gastroenterol 2012;18:6521-6. [Crossref] [PubMed]
- Rubenstein JH, Shaheen NJ. Epidemiology, Diagnosis, and Management of Esophageal Adenocarcinoma. Gastroenterology 2015;149:302-17.e1. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Domper Arnal MJ, Ferrandez Arenas A, Lanas Arbeloa A. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol 2015;21:7933-43. [Crossref] [PubMed]
- Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013;19:5598-606. [Crossref] [PubMed]
- Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol 2013;23:3-9. [Crossref] [PubMed]
- Lepage C, Drouillard A, Jouve JL, et al. Epidemiology and risk factors for oesophageal adenocarcinoma. Dig Liver Dis 2013;45:625-9. [Crossref] [PubMed]
- Lofdahl HE, Lu Y, Lagergren P, et al. Risk factors for esophageal adenocarcinoma after antireflux surgery. Ann Surg 2013;257:579-82. [Crossref] [PubMed]
- Pophali P, Halland M. Barrett's oesophagus: diagnosis and management. Bmj 2016;353:i2373. [Crossref] [PubMed]
- Macias-Garcia F, Dominguez-Munoz JE. Update on management of Barrett's esophagus. World J Gastrointest Pharmacol Ther 2016;7:227-34. [Crossref] [PubMed]
- Spechler SJ, Fitzgerald RC, Prasad GA, et al. History, molecular mechanisms, and endoscopic treatment of Barrett's esophagus. Gastroenterology 2010;138:854-69. [Crossref] [PubMed]
- Noffsinger AE. Defining Cancer Risk in Barrett's Esophagus: A Pathologist's Perspective. Gastrointest Cancer Res 2008;2:308-10. [PubMed]
- Spechler SJ, Souza RF. Barrett's esophagus. N Engl J Med 2014;371:836-45. [Crossref] [PubMed]
- Prasad GA, Bansal A, Sharma P, et al. Predictors of progression in Barrett's esophagus: current knowledge and future directions. Am J Gastroenterol 2010;105:1490-502. [Crossref] [PubMed]
- Mawhinney MR, Glasgow RE. Current treatment options for the management of esophageal cancer. Cancer Manag Res 2012;4:367-77. [PubMed]
- Bollschweiler E, Holscher AH, Schmidt M, et al. Neoadjuvant treatment for advanced esophageal cancer: response assessment before surgery and how to predict response to chemoradiation before starting treatment. Chin J Cancer Res 2015;27:221-30. [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Tan C, Qian X, Guan Z, et al. Potential biomarkers for esophageal cancer. Springerplus 2016;5:467. [Crossref] [PubMed]
- Fatehi Hassanabad A, Chehade R, Breadner D, et al. Esophageal carcinoma: Towards targeted therapies. Cell Oncol (Dordr) 2020;43:195-209. [Crossref] [PubMed]
- Nagaraja V, Shaw N, Morey AL, et al. HER2 expression in oesophageal carcinoma and Barrett's oesophagus associated adenocarcinoma: An Australian study. Eur J Surg Oncol 2016;42:140-8. [Crossref] [PubMed]
- Yang QS, Jiang LP, He CY, et al. Up-Regulation of MicroRNA-133a Inhibits the MEK/ERK Signaling Pathway to Promote Cell Apoptosis and Enhance Radio-Sensitivity by Targeting EGFR in Esophageal Cancer In Vivo and In Vitro. J Cell Biochem 2017;118:2625-34. [Crossref] [PubMed]
- Ladeira K, Macedo F, Longatto-Filho A, et al. Angiogenic factors: role in esophageal cancer, a brief review. Esophagus 2018;15:53-8. [Crossref] [PubMed]
- Hassan MS, Williams F, Awasthi N, et al. Combination effect of lapatinib with foretinib in HER2 and MET co-activated experimental esophageal adenocarcinoma. Sci Rep 2019;9:17608. [Crossref] [PubMed]
- Kelly RJ. Immunotherapy for Esophageal and Gastric Cancer. Am Soc Clin Oncol Educ Book 2017;37:292-300. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol 2018;19:1372-84. [Crossref] [PubMed]
- Smyth EC, Rowley S, Cafferty FH, et al. Safety and Efficacy of the Addition of Lapatinib to Perioperative Chemotherapy for Resectable HER2-Positive Gastroesophageal Adenocarcinoma: A Randomized Phase 2 Clinical Trial. JAMA Oncol 2019;5:1181-7. [Crossref] [PubMed]
- Meric-Bernstam F, Hamilton EP, Beeram M, et al. Zanidatamab (ZW25) in HER2-expressing gastroesophageal adenocarcinoma (GEA): Results from a phase I study. J Clin Oncol 2021;39:164. [Crossref]
- Shitara K, Bang YJ, Iwasa S, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med 2020;382:2419-30. [Crossref] [PubMed]
- Qi S, Mao Y, Jiang M. A phase I study evaluating combined nimotuzumab and neoadjuvant chemoradiotherapy followed by surgery in locally advanced esophageal cancer. Cancer Chemother Pharmacol 2019;84:1115-23. [Crossref] [PubMed]
- Moehler M, Maderer A, Thuss-Patience PC, et al. Cisplatin and 5-fluorouracil with or without epidermal growth factor receptor inhibition panitumumab for patients with non-resectable, advanced or metastatic oesophageal squamous cell cancer: a prospective, open-label, randomised phase III AIO/EORTC trial (POWER). Ann Oncol 2020;31:228-35. [Crossref] [PubMed]
- Brenner B, Purim O, Gordon N, et al. The addition of cetuximab to preoperative chemoradiotherapy for locally advanced esophageal squamous cell carcinoma is associated with high rate of long term survival: Mature results from a prospective phase Ib/II trial. Radiother Oncol 2019;134:74-80. [Crossref] [PubMed]
- Fuchs CS, Shitara K, Di Bartolomeo M, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:420-35. [Crossref] [PubMed]
- Van Cutsem E, Karaszewska B, Kang YK, et al. A Multicenter Phase II Study of AMG 337 in Patients with MET-Amplified Gastric/Gastroesophageal Junction/Esophageal Adenocarcinoma and Other MET-Amplified Solid Tumors. Clin Cancer Res 2019;25:2414-23. [Crossref] [PubMed]
- Shah MA, Bang YJ, Lordick F, et al. Effect of Fluorouracil, Leucovorin, and Oxaliplatin With or Without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma: The METGastric Randomized Clinical Trial. JAMA Oncol 2017;3:620-7. [Crossref] [PubMed]
- Joshi SS, Maron SB, Catenacci DV. Pembrolizumab for treatment of advanced gastric and gastroesophageal junction adenocarcinoma. Future Oncol 2018;14:417-30. [Crossref] [PubMed]
- Moehler M, Shitara K, Garrido M, et al. LBA6_PR Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann Oncol 2020;31:S1191. [Crossref]
- Almhanna K, Meredith KL, Hoffe SE, et al. Targeting the human epidermal growth factor receptor 2 in esophageal cancer. Cancer Control 2013;20:111-6. [Crossref] [PubMed]
- Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest 2001;19:554-68. [Crossref] [PubMed]
- Iqbal N, Iqbal N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol Biol Int 2014;2014:852748 [Crossref] [PubMed]
- Gowryshankar A, Nagaraja V, Eslick GD. HER2 status in Barrett's esophagus & esophageal cancer: a meta analysis. J Gastrointest Oncol 2014;5:25-35. [PubMed]
- Nagaraja V, Eslick GD. HER2 expression in gastric and oesophageal cancer: a meta-analytic review. J Gastrointest Oncol 2015;6:143-54. [PubMed]
- Davidson M, Starling N. Trastuzumab in the management of gastroesophageal cancer: patient selection and perspectives. Onco Targets Ther 2016;9:7235-45. [Crossref] [PubMed]
- Wagner AD, Grabsch HI, Mauer M, et al. EORTC-1203-GITCG - the "INNOVATION"-trial: Effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of HER2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: a randomized phase II-intergroup trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer group. BMC Cancer 2019;19:494. [Crossref] [PubMed]
- McKeage K, Perry CM. Trastuzumab: a review of its use in the treatment of metastatic breast cancer overexpressing HER2. Drugs 2002;62:209-43. [Crossref] [PubMed]
- Gerson JN, Skariah S, Denlinger CS, et al. Perspectives of HER2-targeting in gastric and esophageal cancer. Expert Opin Investig Drugs 2017;26:531-40. [Crossref] [PubMed]
- Swain SM, Miles D, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 2020;21:519-30. [Crossref] [PubMed]
- Liu T, Qin Y, Li J, et al. Pertuzumab in combination with trastuzumab and chemotherapy for Chinese patients with HER2-positive metastatic gastric or gastroesophageal junction cancer: a subpopulation analysis of the JACOB trial. Cancer Commun (Lond) 2019;39:38. [Crossref] [PubMed]
- Shitara K, Hara H, Yoshikawa T, et al. Pertuzumab plus trastuzumab and chemotherapy for Japanese patients with HER2-positive metastatic gastric or gastroesophageal junction cancer: a subgroup analysis of the JACOB trial. Int J Clin Oncol 2020;25:301-11. [Crossref] [PubMed]
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med 2008;358:1160-74. [Crossref] [PubMed]
- Anvari K, Sima HR, Seilanian Toussi M, et al. EGFR Expression in Patients with Esophageal Squamous Cell Carcinoma and its Association with Pathologic Response to Preoperative Chemoradiotherapy: A Study in Northeastern Iran. Arch Iran Med 2017;20:240-5. [PubMed]
- Kim BJ, Kim JH, Jang HJ, et al. The role of anti-EGFR agents in the first-line treatment of advanced esophago-gastric adenocarcinoma: a meta-analysis. Oncotarget 2017;8:99033-40. [Crossref] [PubMed]
- Salati M, Cascinu S. Anti-EGFR therapy in oesophagogastric cancer: precise but not enough. Ann Oncol 2018;29:1884-5. [Crossref] [PubMed]
- Zhang X, Jia J, Lu M, et al. Nimotuzumab Plus Paclitaxel and Cisplatin as a 1(st)-Line Treatment for Esophageal Cancer: Long Term Follow-up of a Phase II Study. J Cancer 2019;10:1409-16. [Crossref] [PubMed]
- Malka D, François E, Penault-Llorca F, et al. FOLFOX alone or combined with rilotumumab or panitumumab as first-line treatment for patients with advanced gastroesophageal adenocarcinoma (PRODIGE 17-ACCORD 20-MEGA): a randomised, open-label, three-arm phase II trial. Eur J Cancer 2019;115:97-106. [Crossref] [PubMed]
- Huang ZH, Ma XW, Zhang J, et al. Cetuximab for esophageal cancer: an updated meta-analysis of randomized controlled trials. BMC Cancer 2018;18:1170. [Crossref] [PubMed]
- Gibson MK, Catalano P, Kleinberg LR, et al. Phase II Study of Preoperative Chemoradiotherapy with Oxaliplatin, Infusional 5-Fluorouracil, and Cetuximab Followed by Postoperative Docetaxel and Cetuximab in Patients with Adenocarcinoma of the Esophagus: A Trial of the ECOG-ACRIN Cancer Research Group (E2205). Oncologist 2020;25:e53-e59. [Crossref] [PubMed]
- Ruhstaller T, Thuss-Patience P, Hayoz S, et al. Neoadjuvant chemotherapy followed by chemoradiation and surgery with and without cetuximab in patients with resectable esophageal cancer: a randomized, open-label, phase III trial (SAKK 75/08). Ann Oncol 2018;29:1386-93. [Crossref] [PubMed]
- Roskoski R Jr. Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit Rev Oncol Hematol 2007;62:179-213. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Gao CF, Vande Woude GF. HGF/SF-Met signaling in tumor progression. Cell Res 2005;15:49-51. [Crossref] [PubMed]
- Creemers A, Ebbing EA, Pelgrim TC, et al. A systematic review and meta-analysis of prognostic biomarkers in resectable esophageal adenocarcinomas. Sci Rep 2018;8:13281. [Crossref] [PubMed]
- Paterson AL, O'Donovan M, Provenzano E, et al. Characterization of the timing and prevalence of receptor tyrosine kinase expression changes in oesophageal carcinogenesis. J Pathol 2013;230:118-28. [Crossref] [PubMed]
- Al-Batran SE, Werner D. Recent advances and future trends in the targeted therapy of metastatic gastric cancer. Expert Rev Gastroenterol Hepatol 2014;8:555-69. [Crossref] [PubMed]
- Mesteri I, Schoppmann SF, Preusser M, et al. Overexpression of CMET is associated with signal transducer and activator of transcription 3 activation and diminished prognosis in oesophageal adenocarcinoma but not in squamous cell carcinoma. Eur J Cancer 2014;50:1354-60. [Crossref] [PubMed]
- Hughes PE, Rex K, Caenepeel S, et al. In Vitro and In Vivo Activity of AMG 337, a Potent and Selective MET Kinase Inhibitor, in MET-Dependent Cancer Models. Mol Cancer Ther 2016;15:1568-79. [Crossref] [PubMed]
- Shah MA, Cho JY, Tan IB, et al. A Randomized Phase II Study of FOLFOX With or Without the MET Inhibitor Onartuzumab in Advanced Adenocarcinoma of the Stomach and Gastroesophageal Junction. Oncologist 2016;21:1085-90. [Crossref] [PubMed]
- Catenacci DVT, Tebbutt NC, Davidenko I, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1467-82. [Crossref] [PubMed]
- Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 2018;18:153-67. [Crossref] [PubMed]
- Available online: https://www.integratedoncology.com/test-menu/43831/pd-l1-by-ihc-22c3-keytruda%C2%AE, accessed 02/23/2021.
- Fashoyin-Aje L, Donoghue M, Chen H, et al. FDA Approval Summary: Pembrolizumab for Recurrent Locally Advanced or Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma Expressing PD-L1. Oncologist 2019;24:103-9. [Crossref] [PubMed]
- Shah MA, Kojima T, Hochhauser D, et al. Efficacy and Safety of Pembrolizumab for Heavily Pretreated Patients With Advanced, Metastatic Adenocarcinoma or Squamous Cell Carcinoma of the Esophagus: The Phase 2 KEYNOTE-180 Study. JAMA Oncol 2019;5:546-50. [Crossref] [PubMed]
- Kojima T, Shah MA, Muro K, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol 2020;38:4138-48. [Crossref] [PubMed]
- Kato K, Sun JM, Shah MA, et al. LBA8_PR Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase 3 KEYNOTE-590 study. Ann Oncol 2020;31:S1192-3. [Crossref]
- Kelly RJ, Ajani JA, Kuzdzal J, et al. LBA9_PR Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer (EC/GEJC) following neoadjuvant chemoradiation therapy (CRT): First results of the CheckMate 577 study. Ann Oncol 2020;31:S1193-4. [Crossref]
- Catenacci DV, Rosales M, Chung HC, et al. MAHOGANY: margetuximab combination in HER2+ unresectable/metastatic gastric/gastroesophageal junction adenocarcinoma. Future Oncol 2021;17:1155-64. [Crossref] [PubMed]
- Janjigian YY, Maron SB, Chatila WK, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol 2020;21:821-31. [Crossref] [PubMed]
- Tintelnot J, Goekkurt E, Binder M, et al. Ipilimumab or FOLFOX with Nivolumab and Trastuzumab in previously untreated HER2-positive locally advanced or metastatic EsophagoGastric Adenocarcinoma - the randomized phase 2 INTEGA trial (AIO STO 0217). BMC Cancer 2020;20:503. [Crossref] [PubMed]
- Moentenich V, Gebauer F, Comut E, et al. Claudin 18.2 expression in esophageal adenocarcinoma and its potential impact on future treatment strategies. Oncol Lett 2020;19:3665-70. [Crossref] [PubMed]
- Sahin U, Türeci Ö, Manikhas G, et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol 2021;32:609-19. [Crossref] [PubMed]
- Shah M, Ajani JA, Al-Batran SE, et al. 836TiP - GLOW: Randomized phase III study of zolbetuximab + CAPOX compared with placebo + CAPOX as first-line treatment of patients with CLD18.2+/HER2− locally advanced unresectable or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma. Ann Oncol 2019;30:v322. [Crossref]
- Yamaguchi K, Shitara K, Al-Batran SE, et al. 198TiP - SPOTLIGHT: Comparison of zolbetuximab or placebo + mFOLFOX6 as first-line treatment in patients with claudin18.2+/HER2– locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma (GEJ): A randomized phase III study. Ann Oncol 2019;30:ix66-ix67. [Crossref]
- Tokunaga R, Imamura Y, Nakamura K, et al. Fibroblast growth factor receptor 2 expression, but not its genetic amplification, is associated with tumor growth and worse survival in esophagogastric junction adenocarcinoma. Oncotarget 2016;7:19748-61. [Crossref] [PubMed]
- Merz V, Zecchetto C, Simionato F, et al. A phase II trial of the FGFR inhibitor pemigatinib in patients with metastatic esophageal-gastric junction/gastric cancer trastuzumab resistant: the FiGhTeR trial. Ther Adv Med Oncol 2020;12:1758835920937889 [Crossref] [PubMed]
Cite this article as: Hassan MS, Makuru V, von Holzen U. Targeted therapy in esophageal cancer. Dig Med Res 2021;4:29.


