
Minimally invasive surgical management of gastric malignancies: role of robotic surgery
Background
The adoption of minimally invasive approaches to the treatment of gastric cancer has afforded patients improved care compared to historical open procedures. With the introduction of laparoscopic gastrectomy in the early 1990’s, this minimally invasive technique has proven its superiority over open gastrectomy in the treatment of gastric cancer in numerous prospective and retrospective studies (1-4). Advantages including reduced postoperative pain, shorter hospital stay and decreased morbidity have been achieved, and it has demonstrated non-inferior results in terms of oncological outcomes (specifically adequate surgical margins, lymph node retrieval, disease-free survival and overall survival when compared to open techniques) (5).
The current literature is heavily based on eastern hemisphere experience with few notable studies from the USA. Three countries, Japan, Korea and China, account for 60% of the total cases of gastric cancer. Routine surveillance programs allow for the detection of cancer with a notably high percentage of early-stage gastric cancer resulting in T1a/1b constituting 80% of the patient cohort (6). This has resulted in a superior 5-year survival compared to the USA (27%) and Europe (22%). Oncologic stage at the time of surgery is more advanced in the USA (≥T2 45%) and Europe (>T2N1 55–75%) (2,7). Given the more advanced stage, patients diagnosed are more likely to receive neoadjuvant chemotherapy or chemoradiotherapy (8).
Interest in the role of robotic surgery has been increasing over the last two decades. Following the first report of robotic assisted gastrectomy for gastric cancer in 2002 (9), Giulianotti et al. (10) published the first study analyzing robotic gastrectomy. In this retrospective study including 193 patients who underwent a wide variety of robotic surgeries, fundoplication for gastroesophageal reflux disease was the second most common procedure after cholecystectomy, and required a small learning curve of 20 operations. Robotic surgery seems to be of benefit in procedures where fine dissection and high precision are required. Though subsequent studies to compare robotic gastrectomy to laparoscopy have highlighted the benefits of this technique, the superiority of the robot assisted procedure has not widely been demonstrated. In this review, we aim to clarify the present and the future of gastric cancer surgery.
The present in gastric minimally invasive techniques
Minimally invasive techniques offer the patient endoscopic approaches or comparable surgery through small incisions which aid in a faster recovery, less postoperative pain and shorter hospital stay. The laparoscopic approach is beginning to be replaced by endoscopic or robotic assisted techniques. Endoscopy has evolved from a diagnostic tool to a therapeutic alternative for early stage (cTis or cT1a) gastric cancer in which endoscopic mucosal resection (EMR) has proven to be effective (11,12). The advantages of robotic surgery are becoming more compelling when considering a highly technical procedure such as gastrectomy for gastric cancer which includes a D2 lymphadenectomy (13).
Therefore, we searched for prospective and retrospective articles comparing laparoscopic and robotic gastrectomy for the last 5 years. We found ten articles on this subject from which 6 correspond to Asian countries, 3 to Europe and 1 to the USA. Only one article by Kim et al. (14) is a prospective multicenter study, though it is non-randomized. Herein we review the current and most recent evidence concerning benefits of robotic-assisted gastrectomy (Table 1).
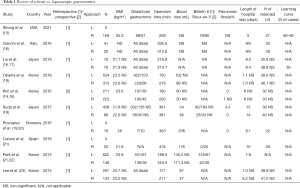
Full table
Tumor stage and location
Most authors agree on the indication of laparoscopic or robotic gastrectomy for early gastric cancer with tumors with a clinical stage lower than T2N1M0 (4,24). Surgical approach for more advanced gastric cancer is still a subject of controversy, and surgeons seem to opt for a “more secure”, open gastrectomy when indicating surgery for large tumors, infiltration of serosa or infiltration of neighboring organs. In a retrospective, single-center, single-surgeon study comparing laparoscopic vs. robotic gastrectomy, Kim et al. (25) show more advanced T classification and N classification in the robotic group than in the laparoscopic group without associated adverse negative outcomes. Concerning the location of tumors in gastric cancer, recently published studies compared laparoscopic vs. robotic distal gastrectomy (16,17,23), while the remaining studies compared results for both total and distal gastrectomy, although the ratio distal:total favors distal gastrectomy in all of them. This is probably due to the complexity of total gastrectomy, which is usually performed with an open procedure.
Patients’ body mass index
In accordance with the literature, studies from Asia reviewed in this article show mean body mass index of 23 kg/m2. Surprisingly, the article from Procopiuc et al. (20) reports a mean BMI of 26.05 km/m2, which is higher than the mean BMI of 25.8 reported by Strong et al. (15) from a population of the U.S.A. However, the range of BMI from the European study ranges from 22 to 32 kg/m2, whereas the range reported by Strong et al. is wide as 16.9–44.9 kg/m2. Besides the fact that robotic gastrectomies are common in Asian countries due to the prevalence of this disease, some authors affirm that the use of the robot could be advantageous in gastrectomies in patients with higher BMI, due to the confined space and smaller visual field for dissection in these individuals (23).
Learning curves
In a study comparing learning curves for laparoscopic and robotic gastrectomy by Kim et al. (25), 172 robotic and 481 laparoscopic distal gastrectomies were performed by the same surgeon who achieved stable operation time at 95 robotic and 270 laparoscopic gastrectomies, with no differences in surgical outcomes. Alhossaini et al. (6) reported a learning curve of 11–25 cases for robotic gastrectomy for experienced gastric surgeons, while 40–60 are said to be required to attain the same standards in laparoscopic gastrectomy. Moreover, Huang et al. (4) examined the performance by a single surgeon, who demonstrated a significant reduction in operative time after the initial 25 cases of robotic gastrectomy, while there was no such trend for laparoscopic gastrectomy. These results suggest that learning curves are shorter for robotic procedures.
Intraoperative bleeding
Due to the rich arterial blood supply to the stomach and the proximity to major splanchnic vessels, distal and total gastrectomies are operations with the potential for hemorrhagic complications. Concerning this, the robot poses an advantage over conventional laparoscopy, as it enables a highly accurate dissection due to its three-dimensional magnified vision, with 7 degrees of freedom and enhanced wrist movements. Reduced blood loss is the perioperative outcome which has demonstrated an advantage of the robot in numerous studies on robotic gastrectomy (1,6,23,26), as well as other surgical procedures.
Mean operation time
Longer operation time is one of the most evident disadvantages of robot surgery. Surgical time required for setup, docking, and instrument exchanges counter the many benefits of the technology. Operation time with the robot is at least one hour greater than that of laparoscopic gastrectomy, making the procedure “less efficient”. Liu et al. (17) published an article in 2018 in which videos on laparoscopic and robotic gastrectomies were revised to determine the time consumed at each step of these procedures. In this study, the effective time of surgery was only 15.3 minutes longer in the robotic procedure than in the laparoscopic gastrectomy. The remaining excessive time in robotic procedures (described as “junk time”) attributed to setting up, docking, adjusting the surgical instruments, and instrument changes, produced an overall operation time of 56.8 minutes longer for robotic gastrectomy as compared to conventional laparoscopy. However, as demonstrated by Huang et al. (4), the docking time can be progressively reduced with experience.
Pancreatic fistula
Pancreatic fistula rates vary between reported retrospective and prospective studies. Factors associated with pancreatic fistula are related to the operative procedure, the stage of gastric cancer, and prior treatment with chemotherapy. Neoadjuvant chemotherapy has shown a statistically significant increased rate of pancreatic fistula when compared to surgery alone (14.7% vs. 3.3%; P=0.011), in a retrospective study by Kosaka et al. (27). In addition, a greater extent of lymphadenectomy (which may include bursectomy) and the addition of an associated procedure such as splenectomy or pancreatectomy has shown rates of 30% of postoperative pancreatic fistula.
In D2 lymphadenectomy together with a total or distal gastrectomy, the incidence of pancreatic fistula lies between 0% and 6% (28). Although pancreatic fistula is a rare complication of gastrectomy, its potentially serious consequences should not be underestimated. A meta-analysis by Guerra et al. (29) showed a trend towards fewer pancreatic complications such as pancreatic fistula for robotic-assisted when compared to laparoscopic gastrectomy, although without statistical significance. In a retrospective study by Suda et al. (19), 88 robotic and 438 laparoscopic radical gastrectomies were compared, and a decrease in local postoperative complications was reported in the robotic group, specifically a reduction in the incidence of pancreatic fistula from 4.3% to 0%. Moreover, morbidity and mortality of the laparoscopic group were 11.4% and 0.2%, respectively, whereas those of the robotic group were 2.3% and 1.1%. This is the first study showing that non-use of the surgical robot was the most important factor related to the complications.
Length of hospital stay
With the implementation of minimally invasive techniques, the goal was to achieve a faster recovery after surgery, with reduced analgesic use and incision-related complications (wound infection/hernia/evisceration). Faster recovery leads to an earlier oral tolerance, reduced length of hospital stay, and faster return to normal activities. These results are the reason for the widespread implementation of minimally invasive surgery.
Robotic surgery has already proven better outcomes in terms of lower analgesic consumption, faster recovery and shorter length of hospital stay in Urology and Gynecology, surgical fields in which the robot is already extensively implemented (30). In robotic gastrectomy for gastric cancer, studies show similar figures for both techniques and only the retrospective study by Suda et al. (19) has shown a reduction by 1 day of hospital stay favoring the robotic approach (although one patient in the laparoscopic group had a hospital stay of 136 days). Increased experience and applicability of the robot, and prospective randomized trials will hopefully bring more light as regards the advantages of this least invasive technique.
Number of retrieved lymph nodes
Lymphatic drainage of the stomach was classified into 16 lymph node stations by the Japanese Research Society for Gastric Cancer (JRSGC) in 1973 (31). Later, in 2011, the Japanese Gastric Cancer Association (JGCA) categorized anatomically and labelled numerically the lymph node stations. The extent of lymphadenectomy was stated to depend on the type of gastric resection. Therefore, for a total gastrectomy: D1 included lymph node levels 1 to 7, D1+ included D1 plus levels 8a, 9 and 11p, D2 included D1+ plus stations 10, 11d, 12a. For tumors invading the esophagus, D1+ includes level 110 and D2 levels 19, 20, 110 and 111. For distal tumors, D1 are stations 1, 3, 4sb, 4d, 5, 6 and 7; D1+ includes stations 8a and 9; D2 includes D1+ plus 11p and 12a (Table 2). JGCA suggests D1 or D1+ for early gastric cancer not suitable for EMR with clinically negative lymph nodes, and D2 if suspicious nodes are present.
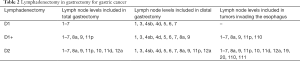
Full table
This recommendation varies for the Western countries, as they are prone to encounter more advanced gastric cancers. In this context, guidelines advise a D2 lymphadenectomy for early gastric cancer not suitable for EMR (32).
Some authors believe an extended lymphadenectomy results in better loco-regional control, leading to improvements in survival, whilst others believe it only increases the morbidity of the procedure. An ideal equilibrium between improved survival and decreased morbidity may be achieved once the learning curve for robotic surgery is reached. Cianchi et al. (16) have reported a greater number of retrieved lymph nodes for robotic than laparoscopic gastrectomy with statistical significance, without compromising the safety of the procedure. This proficiency is believed to be related to the enhanced endo-wrist movements of the robot which allow for a meticulous and non-traumatic dissection (6,26).
Oncological outcomes
Oncological outcomes in terms of compromised margins, 5-year survival, overall survival, and disease-free survival for robotic gastrectomy are reported to be at least comparable to those of laparoscopic gastrectomy (6,7,33). Obama et al. (18) published the largest single-center retrospective series analyzing overall-survival and relapse-free survival and found no differences between robotic and laparoscopic gastrectomy. However, results may be biased by patient selection, and randomized controlled trials are still awaited to better address these results.
The future
What awaits us in the future of surgical management of gastric malignancy?
Endosonography and interventional endoscopy have evolved into the treatment of choice for early gastric cancer with tumors infiltrating no deeper than submucosa. This became possible due to advances in EMR such as the insulated-tip electrosurgical knife, which enables an en-bloc resection of the tumor without compromising its margins. Even so, laparoscopic intra-gastric resection has been implemented by some authors as a minimally invasive technique to treat certain lesions of early gastric cancer which are larger (>1.5 cm) and/or more difficult to access (in the fundus, posterior stomach wall or at the GE junction) (11).
The experience with the robot is expanding worldwide at a fast speed. Newer versions of the Da Vinci (Intuitive Surgical Inc., Sunnyvale, CA, USA), with improved operating tools, are constantly emerging to overcome even modest limitations of the apparatus. Instruments are progressively narrower, longer and offer increased rotation and movement capability. Stapling devices and vessel sealers are all becoming more efficient. Yet, studies comparing the existing robots are needed to better determine their surgical efficiency and limitations.
The fusion of minimally invasive surgery and localized imaging seems promising. Indocyanine-green assisted intraoperative fluorescence has been described to guide tumor location, vessel perfusion and lymphatic drainage in many surgical fields. Image-guided surgery with anatomical reconstruction based on preoperative CT scan or MRI is widely advanced in Neurosurgery and Orthopedics. Although these devices have not been widely applied to gastric surgery, we believe that combined techniques are undoubtedly the future.
Discussion
We are presently experiencing an era of accelerated technological advance with a subsequent boom in minimally invasive techniques. Meanwhile, although robotic surgery has shown non-inferior results in terms of perioperative outcomes, its high costs and increased operation time make it less appealing.
Certain aspects about the available information on robotic gastrectomy need to be addressed. As discussed in this review, multiple studies suggest that robotic assisted gastrectomy is associated with decreased blood loss, decreased hospital stay, decreased complications (pancreatic fistula), and increased number of lymph nodes retrieved in the treatment of gastric malignancy (16,19,23). The improved visualization and precision of dissection of the robot could lead to a reduction in tissue trauma, which in turn decreases blood loss and local complications, and in turn reduces time of recovery and hospital stay. However, its costs and operation time continue to raise apprehension among surgeons and health systems. This pitfall may make the robot a less attractive approach for less complex and shorter procedures.
On this matter, a great contribution was made by Alhossaini et al. in their review of 2017. Perioperative outcomes were analyzed for a group of surgeons, comparing their initial robotic gastrectomies to the procedures performed later, at a Korean center where more than 1,000 performed robotic assisted gastrectomies have been performed. This study provided rich information as it showed that after an initial experience with the robot, better (and statistically significant) outcomes, such as decreased operative time and increased number of retrieved lymph nodes are obtained. This implies we are just witnessing the learning curve of robotic-assisted gastric surgery, and time is needed for surgeons and health care facilities to adapt to this contemporary device. With practice, set up and docking system times can be reduced, making operative times more comparable to laparoscopic gastrectomy.
It is important to highlight that most articles on robotic gastrectomy involve Asian populations, who are known to have the highest incidence of gastric cancer in earlier stages and lower body mass indexes. Higher BMI is associated with a higher percentage of intra-abdominal fat that may impair optimal exposure of anatomical structures, even more in tumors of the gastroesophageal junction (11). We hope to find greater advantages in the use of the robot in these populations, given that a greater BMI and a greater tumor stage often translate to a more complex operation, and this can be overcome with the greater precision and visual magnification, reduced tremor, greater range of view and more grades of freedom offered by the robot. Hence, additional studies on western populations, who have higher BMI, more prevalence of cardia gastric cancer and more advanced gastric tumors are necessary. Most importantly, randomized trials demonstrating equivalent oncologic outcomes with the use of robotic assisted techniques for gastric cancer must be performed.
The information hereby discussed suggests that even though research establishing the superiority of robotic assisted gastrectomy is lacking, the advantages of the procedure will likely translate to better outcomes for patients with gastric adenocarcinoma. Ultimately, as tools such as real-time tomographic and endoscopic imaging, near infrared imaging and anatomical mapping evolve into a hybrid surgical therapeutic modality, advantages of the robot will see no limits (6,9,34).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alfredo Daniel Guerron) for the series “Advanced Laparoscopic Gastric Surgery” published in Digestive Medicine Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-21-12. The series “Advanced Laparoscopic Gastric Surgery” was commissioned by the editorial office without any funding or sponsorship. All authors have no other conflicts of interest to declare.
Ethical Statement: All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kelly KJ, Selby L, Chou JF, et al. Laparoscopic Versus Open Gastrectomy for Gastric Adenocarcinoma in the West: A Case-Control Study. Ann Surg Oncol 2015;22:3590-6. [Crossref] [PubMed]
- Strong VE, Devaud N, Allen PJ, et al. Laparoscopic Versus Open Subtotal Gastrectomy for Adenocarcinoma: A Case-Control Study. Ann Surg Oncol 2009;16:1507-13. [Crossref] [PubMed]
- Haverkamp L, Brenkman HJF, Seesing MFJ, et al. Laparoscopic versus open gastrectomy for gastric cancer, a multicenter prospectively randomized controlled trial (LOGICA-trial). BMC Cancer 2015;15:556. [Crossref] [PubMed]
- Huang KH, Lan YT, Fang WL, et al. Initial experience of robotic gastrectomy and comparison with open and laparoscopic gastrectomy for gastric cancer. J Gastrointest Surg 2012;16:1303-10. [Crossref] [PubMed]
- Cui M, Li Z, Xing J, et al. A prospective randomized clinical trial comparing D2 dissection in laparoscopic and open gastrectomy for gastric cancer. Med Oncol 2015;32:241. [Crossref] [PubMed]
- Alhossaini RM, Altamran AA, Seo WJ, et al. Robotic gastrectomy for gastric cancer: Current evidence. Ann Gastroenterol Surg 2017;1:82-9. [Crossref] [PubMed]
- van Boxel GI, Ruurda JP, van Hillegersberg R. Robotic-assisted gastrectomy for gastric cancer: a European perspective. Gastric Cancer 2019;22:909-19. [Crossref] [PubMed]
- Siewert JR, Rüdiger Siewert J. Gastric cancer: the dispute between East and West. Gastric Cancer 2005;8:59-61. [Crossref] [PubMed]
- Hashizume M, Shimada M, Tomikawa M, et al. Early experiences of endoscopic procedures in general surgery assisted by a computer-enhanced surgical system. Surg Endosc 2002;16:1187-91. [Crossref] [PubMed]
- Giulianotti PC. Robotics in General Surgery. Arch Surg 2003;138:777. [Crossref] [PubMed]
- Rosen MJ, Heniford BT. Endoluminal gastric surgery: the modern era of minimally invasive surgery. Surg Clin North Am 2005;85:989-1007. vii. [Crossref] [PubMed]
- Ono H. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001;48:225-9. [Crossref] [PubMed]
- Shen WS, Xi HQ, Chen L, et al. A meta-analysis of robotic versus laparoscopic gastrectomy for gastric cancer. Surg Endosc 2014;28:2795-802. [Crossref] [PubMed]
- Kim HI, Han SU, Yang HK, et al. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg 2016;263:103-9. [Crossref] [PubMed]
- Strong VE, Russo AE, Nakauchi M, et al. Robotic Gastrectomy for Gastric Adenocarcinoma in the USA: Insights and Oncologic Outcomes in 220 Patients. Ann Surg Oncol 2021;28:742-50. [Crossref] [PubMed]
- Cianchi F, Indennitate G, Trallori G, et al. Robotic vs laparoscopic distal gastrectomy with D2 lymphadenectomy for gastric cancer: a retrospective comparative mono-institutional study. BMC Surg 2016;16:65. [Crossref] [PubMed]
- Liu H, Kinoshita T, Tonouchi A, et al. What are the reasons for a longer operation time in robotic gastrectomy than in laparoscopic gastrectomy for stomach cancer? Surg Endosc 2019;33:192-8. [Crossref] [PubMed]
- Obama K, Kim YM, Kang DR, et al. Long-term oncologic outcomes of robotic gastrectomy for gastric cancer compared with laparoscopic gastrectomy. Gastric Cancer 2018;21:285-95. [Crossref] [PubMed]
- Suda K, Man IM, Ishida Y, et al. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc 2015;29:673-85. [Crossref] [PubMed]
- Procopiuc L, Tudor S, Manuc M, et al. Open vs robotic radical gastrectomy for locally advanced gastric cancer. Int J Med Robot 2016;12:502-8. [Crossref] [PubMed]
- Caruso R, Vicente E, Quijano Y, et al. Robotic assisted gastrectomy compared with open resection: a case-matched study. Updates Surg 2019;71:367-73. [Crossref] [PubMed]
- Park JY, Ryu KW, Reim D, et al. Robot-assisted gastrectomy for early gastric cancer: is it beneficial in viscerally obese patients compared to laparoscopic gastrectomy? World J Surg 2015;39:1789-97. [Crossref] [PubMed]
- Lee J, Kim YM, Woo Y, et al. Robotic distal subtotal gastrectomy with D2 lymphadenectomy for gastric cancer patients with high body mass index: comparison with conventional laparoscopic distal subtotal gastrectomy with D2 lymphadenectomy. Surg Endosc 2015;29:3251-60. [Crossref] [PubMed]
- Hashizume M, Sugimachi K. Robot-assisted gastric surgery. Surg Clin North Am 2003;83:1429-44. [Crossref] [PubMed]
- Kim HI, Park MS, Song KJ, et al. Rapid and safe learning of robotic gastrectomy for gastric cancer: Multidimensional analysis in a comparison with laparoscopic gastrectomy. Eur J Surg Oncol 2014;40:1346-54. [Crossref] [PubMed]
- Yang SY, Roh KH, Kim YN, et al. Surgical Outcomes After Open, Laparoscopic, and Robotic Gastrectomy for Gastric Cancer. Ann Surg Oncol 2017;24:1770-7. [Crossref] [PubMed]
- Kosaka T, Akiyama H, Makino H, et al. Impact of Neoadjuvant Chemotherapy Among Patients with Pancreatic Fistula After Gastrectomy for Advanced Gastric Cancer. Anticancer Res 2016;36:1773-7. [PubMed]
- Washio M, Yamashita K, Niihara M, et al. Postoperative pancreatic fistula after gastrectomy for gastric cancer. Ann Gastroenterol Surg 2020;4:618-27. [Crossref] [PubMed]
- Guerra F, Giuliani G, Formisano G, et al. Pancreatic Complications After Conventional Laparoscopic Radical Gastrectomy Versus Robotic Radical Gastrectomy: Systematic Review and Meta-Analysis. J Laparoendosc Adv Surg Tech A 2018;28:1207-15. [Crossref] [PubMed]
- Giri S, Sarkar DK. Current status of robotic surgery. Indian J Surg 2012;74:242-7. [Crossref] [PubMed]
- . The general rules for The gastric cancer study in surgery. Jpn J Surg 1973;3:61-71. [Crossref] [PubMed]
- Degiuli M, De Manzoni G, Di Leo A, et al. Gastric cancer: Current status of lymph node dissection. World J Gastroenterol 2016;22:2875-93. [Crossref] [PubMed]
- Kim YM, Son T, Kim H-I, et al. Robotic D2 Lymph Node Dissection During Distal Subtotal Gastrectomy for Gastric Cancer: Toward Procedural Standardization. Ann Surg Oncol 2016;23:2409-10. [Crossref] [PubMed]
- Marescaux J, Diana M. Inventing the future of surgery. World J Surg 2015;39:615-22. [Crossref] [PubMed]
Cite this article as: Vanetta C, Lidsky M, Herbert GS, Shah KN, Zani S. Minimally invasive surgical management of gastric malignancies: role of robotic surgery. Dig Med Res 2021;4:34.


