
Focal nodular hyperplasia misdiagnosed as hepatocellular carcinoma: a case report and literature review
Introduction
Focal nodular hyperplasia (FNH) is a rare benign disease of the liver and the second most common benign lesion in healthy, young and middle-aged women (1-4). The pathogenesis of this disease is unclear (5,6). Most researchers think that it is a reactive process of the liver to vascular damage or deformity. The typical feature is that a large artery in the central fibrous scar is present, but the portal vein structure is absent (7). As FNH is rare and has atypical presentation, it is easily misdiagnosed as HCC or hepatic adenoma on imaging or in clinical practice and pathology because both types of lesions show arterial-phase enhancement. Although imaging modalities have important advantages in the diagnosis of benign and malignant tumors of the liver, and liver biopsy is the gold standard for diagnosis of liver diseases, a definitive diagnostic method for hepatic tumors remains to be established, especially when the tumor is large or with multiple lesions (8). Complications such as rupture and bleeding are extremely rare. It has been previously reported that atypical FNH presenting as acute abdomen and misdiagnosed as HCC caused by tumor spontaneous rupture and hemorrhage (9). Here, we report a patient with FNH that was misdiagnosed as HCC and hepatic peliosis, respectively, by imaging modalities and liver biopsy. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/dmr-20-157).
Case presentation
Chief complaints
A 48-year-old woman was admitted to our Department of Gastroenterology because of intermittent upper abdominal discomfort for >1 year on July 25, 2016. The patient presented with a feeling of distension, no obvious abdominal pain, nausea and vomiting, accompanied by yellowing of the skin and sclera. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
History of present illness
Her medical history included intermittent use of oral contraceptives for the past 20 years (dose was unknown). She denied a history of hypertension, diabetes, coronary heart disease, hepatitis or tuberculosis, residence of pastoral area, surgery or trauma. She also denied a family history of cancer. Her hemodynamic status on admission was stable. There was no relevant interventions treatment in the past.
Physical examination
No obvious abnormality was found in the heart and lungs; the upper abdomen was full; no gastrointestinal peristalsis waves were found; the abdomen was soft, without tenderness and rebound pain; Visible mass is palpable in the upper abdomen; the boundary at 5 cm below the liver costal margin and 7 cm below the xiphoid process was not clear; no percussion pain in the liver and kidney regions; negative shifting dullness; bowel sounds 4 times/min.
Laboratory examination
Liver function, routine blood tests, blood coagulation index and tumor markers were nearly normal. Liver function: aspartate transaminase 23 U/L, alanine aminotransferase 20 U/L, alkaline phosphatase 123 U/L, albumin 43.4 g/L, total bilirubin 11.4 μmol/L, direct bilirubin 3.7 μmol/L, cholinesterase 5,405 U/L, γ-glutamyl transferase 136 U/L, prothrombin time 10.3 s, activated partial thromboplastin time 22.9 s, fibrinogen degradation products 5.8 μg/mL, D-dimer 2.444 μg/mL. Routine blood tests: white blood cells 6.39×109/L, blood platelets 229×109/L, red blood cells 3.9×1012/L, hemoglobin 125 g/L. Tumor markers: carcinoembryonic antigen: 1.16 ng/mL, α-fetoprotein 1.52 ng/mL, carbohydrate antigen 19-9 32.84 U/mL, carbohydrate antigen 125 21.13 U/mL. Hepatitis virus antibodies were all negative.
Imaging examination
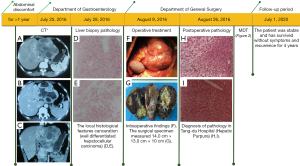
Dynamic abdominal computed tomography (CT) revealed a 13.8 cm × 13.3 cm × 9.8 cm mass of irregular patchy low density with fuzzy boundaries that originated from the lateral segment of the liver left lobe. Other multiple spots of calcareous density were seen in the mass. The tumor exhibited inhomogeneous enhancement; contrast agent in the portal venous phase and delayed phase did not obviously subside; and adjacent stomach, pancreas, and hepatic arteries were compressed by the tumor. The tumor contained a low-density area that was considered to be hepatic cystadenoma and cholangiocarcinoma, which should to be excluded by needle biopsy (Figure 1A,B,C).
Further diagnostic work-up
The patient underwent a percutaneous liver biopsy on July 28, 2016. Pathological examination of biopsied liver tissue showed necrosis of liver cells and the cytoplasm was loose and turbid, and some of the liver cells had dysplasia. The local histological features could not rule out the possibility of canceration of a few liver cells (well-differentiated HCC) (Figure 1D,E).
Final diagnosis
The patient was diagnosed with HCC and transferred to our Department of General Surgery.
Treatment and outcome
The patient underwent resection of the left lobe of the liver under general anesthesia on August 9, 2016. Normal liver size and moderate proportions were found during the operation. The texture was fragile and soft, showing fatty-liver-like changes. Cystic lesions of about 14 cm × 13 cm were seen in the left lobe of the liver, including convex growth, compression of the lesser curvature of the stomach, no invasion and adhesion between the mass and the stomach, irregular septation-like changes on the surface of the mass, the texture was still soft, and the connection with normal liver tissue was more obvious (Figure 1F,G). According to the results of preoperative pathological examination, the anterior approach was selected to perform partial hepatectomy of the left lobe. The operation was successful, and the patient recovered well after the operation.
Postoperative pathological findings
The left lobe of the liver tissue was 15 cm × 9.5 cm × 5.5 cm. There was a palpable, slightly hard, grey-white area, 8 cm × 4.5 cm × 2.5 cm, FNH of the liver with local hepatic steatosis and local hemorrhagic polarization, fibrous nodal tissue hyperplasia, hyaline degeneration and focal calcification, massive blood cavity formation in the liver parenchyma, and histological features suggesting hepatic purpura (Figure 1H,I).
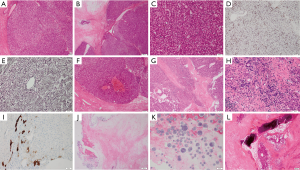
The pathological manifestations were complex, and the tumor had many pathological features of benign liver tumors. Therefore, we conducted a second pathological consultation in Xijing Hospital of our college. Adenomatous hyperplasia of the liver was observed that conformed to the diagnostic criteria for liver adenoma (Figure 2A,B,C). Immunochemistry showed staining of CD34 molecules (Figure 2D) that revealed vascularization of hepatic sinusoidal endothelial cells and reticular tissue (Figure 2E) with incomplete microstructure; therefore, its local pathological features also had the characteristics of hepatic purpura (Figure 2F). The histopathological features of most lesions were consistent with the appearance of FNH, and cytokeratin 7 staining demonstrated histological features of intrahepatic bile duct transition (Figure 2G,H,I) and a large number of areas of tissue necrosis (Figure 2G), crystalline components (Figure 2J,K) and calcification (Figure 2L). After discussion with a multidisciplinary team (MDT), the hepatic tumor was diagnosed as benign FNH. The patient was stable and has survived without symptoms and recurrence for 4 years.
Discussion
FNH is a nontumorous benign nodular disease of the liver and the second most common benign tumor in the liver (7,9-11). Although the disease is not limited by age and sex, it is more common in women aged 20–30 years. Most FNH patients have no clinical symptoms and are diagnosed by imaging and physical examination. Only a small number of patients have atypical clinical symptoms such as right upper abdominal pain, discomfort, nausea, vomiting and other gastrointestinal symptoms. The pathogenesis of this disease is unclear. Most researchers think it is a reactive process of the liver to vascular damage or deformity. The typical features are a large artery in the central fibrous scar and absence of portal vein structure (7).
We report this patient who was misdiagnosed due to preoperative needle biopsy pathology and imaging for the first time. Although imaging modalities have important advantages in the diagnosis of benign and malignant tumors of the liver, and liver biopsy is the gold standard for diagnosis of liver diseases, a definitive diagnostic method for hepatic tumors remains to be established, especially when the tumor is large or with multiple lesions. At the same time, we also emphasize the importance of MDT of difficult pathology. Therefore, I would like to thank the Department of Pathology of Xijing Hospital for its strong support to the pathological diagnosis of this patient. However, this report has many limitations. Firstly, due to full belief in pathological biopsy, no specific magnetic resonance imaging (MRI) was performed before surgery to further clarify the diagnosis. Secondly, we failed to take multiple points during the preoperative liver biopsy, leading to misdiagnosis of pathology, especially for this lager and multiple lesions patient. Finally, for this patient, we did not have the awareness of the differential diagnosis of liver dysplasia nodules during the preoperative diagnosis.
Most liver tumors are malignant (12), especially in China, within the context of hepatitis and cirrhosis. The incidence rate of FNH is low and the clinical diagnosis is difficult, resulting in many clinicians lacking awareness of the disease and being prone to misdiagnosis or mistreatment. As in this patient, preoperative imaging and pathological biopsy suggested that the tumor was HCC, but the final pathological diagnosis was FNH.
Imaging is the most important diagnostic modality, such as color Doppler ultrasound, CT and MRI. Ultrasonography shows no specificity for FNH, and most lesions are hypoechoic. The contrast-enhanced ultrasonography findings of FNHs show a centrifugal spoke-wheel filling pattern in the arterial phase, followed by sustained homogeneous enhancement during the portal venous and late phases. A cinematic loop is recommended to check frame by frame for assessment of the filling pattern (13). Therefore, it is difficult to distinguish FNH from other liver diseases by ultrasonography.
CT of FNH shows localized lesion with low or equal density. Rapid enhancement in arterial lesions can be observed, while the portal delay phase often shows equal density. FNH tumors often show a typical central stellate scar or low density in the arterial phase. The typical central stellate scar shows low density in the dual phase and can strengthen in the delayed phase (14).
It is reported that the sensitivity of MRI for FNH diagnosis is 75% and the specificity is 98% (15,16). The typical performance of MRI is low signal on T1-weighted images, and equal signal or slightly higher signal on T2-weighted images. Contrast enhancement with specific particles, such as gadolinium and super-paramagnetic iron oxide, is helpful to improve the sensitivity and specificity of MRI in the diagnosis of FNH. In recent years, a number of hepatospecific MR contrast agents have become available, such as Gd-BOPTA, Gd-EOB-DTPA, and/or superparamagnetic iron oxide (SPIO)-enhanced MRI. MRI shows low signal intensity, and atypical features for the hepatobiliary phase (17-21). Thus, MRI using a hepatospecific contrast agent may result in better depiction of FNH, but atypical features may complicate specific diagnosis (19,22).
Radionuclide scanning diagnosis of FNH depends on the uptake of radiolabeled sulfur colloids by Kupffer cells of the liver lesions. Normal or increased uptake of radiolabeled 99mTc-sulfur colloids by the lesion contributes to the specific diagnosis of FNH. However, about 50% of the FNH lesions do not take up sulfur colloid, manifested as “cold area” (non-radioactive), so it is difficult to distinguish FNH from other liver parenchymal tumors by radionuclide surface scanning. Therefore, the correct diagnosis of FNH by radionuclide scanning must be combined with medical history and other imaging examinations (23).
The morphology of FNH may sometimes be a challenge for pathologists, particularly when these lesions show histological variations and overlapping features with HCC and other hepatocellular lesions, and particularly when small biopsy samples are evaluated (8,24). Percutaneous liver biopsy can be used in the diagnosis of uncertain liver lesions, when diagnosis by combined imaging examination is still difficult. If a single needle biopsy fails to make a definite diagnosis, it is feasible to perform multiple needle aspirations. However, this is controversial, and some researchers believe that needle biopsy often leads to misdiagnosis because of limited tissue sampling (25). At the same time, FNH is a hypervascularized lesion and is associated with an increased bleeding risk. If it is a malignant tumor, it may lead to metastasis along the needle tract, so it is rarely used clinically. Furthermore, tumor biopsy is associated with a low diagnostic sensitivity, with only 30–45% of all biopsies being consistent with the histology of surgical specimens (26).
FNH treatment depends on the correct diagnosis. Most of the lesions of FNH are small, slow growing, and have a low canceration rate for many years. Therefore, asymptomatic patients with imaging confirmation can be followed up every 6 months. Patients with nonsurgical treatment should be regularly followed up by B ultrasound and CT at least once a year. If the tumor continues to increase or spontaneous rupture and bleeding, it is recommended for surgical resection. If the mass is larger (>5 cm), combined with CT findings, the location of the tumor and distribution of blood flow should be considered comprehensively, and if necessary, transarterial chemoembolization (TACE) is feasible (27,28). Minimally invasive treatment with ethanol injection and radiofrequency ablation (RFA) is suitable for small tumor foci. Although appropriate management is controversial, a lesion strongly suspected of being a malignant tumor may experience rupture and hemorrhage and require surgical resection. However, if the diagnosis is clear, its application is questionable (26,29).
Many researchers believe that clinical symptoms are an indication for surgery for FNH (30). Surgical resection is mostly recommended in patients with obvious clinical symptoms, undiagnosed nodules, and tumor diameter >5 cm. The lesions can be removed and pathological diagnosis can be made. Apart from surgical management of symptomatic FNH, percutaneous radiological modalities have to be considered and include TACE and RFA (26,31,32).
According to the pathological features, FNH can be divided into two types (33). Type 1: classical type, one of the most common, has the structure of regional central stellate fibers with abnormal arterial structure and absence of the portal vein in this area, which contains approximately normal hepatocytes and proliferating cholangial cells. Type 2, accounting for about 19.7%, encompasses cases in which the central region can be seen in the portal vein or the lack of certain structural features typical of FNH, and can be divided into three sub-types (33,34): vascular expansion, adenomatous hyperplasia and atypical large cell types. The clinicopathological features of FNH-like nodules support the hypothesis that vascular alterations in liver cirrhosis play an important role in their pathogenesis and that FNH-like nodules do not have an increased risk of malignant transformation (8). They are sometimes misdiagnosed as HCC on imaging because both types of lesions show arterial-phase atypical enhancement (8,34-36).
Conclusions
Spiral CT and MRI can diagnose FNH correctly in patients with typical features. Liver biopsy is one of the most important methods for definitive diagnosis, but because of limited material, sometimes it may result in misdiagnosis. We should make a reasonable choice in the treatment of FNH, that is, it is necessary to avoid delayed treatment because of missed diagnosis and misdiagnosis, especially for patients with malignant liver lesions or potential malignant risks, and to avoid unnecessary surgical treatment. This case report demonstrates that, despite improved diagnostic methods, there remain some hepatic lesions that cannot be reliably identified by imaging. For those uncertain diagnoses, especially in patients with suspected hepatocellular adenoma or a family history of malignant tumors, marked tumor enlargement and/or jaundice, bleeding and other complications, surgical resection should be considered. In case of symptomatic liver lesions, surgical resection should only be indicated in patients with tumor-specific symptoms (26,30,37). Liver resection is safe and effective for benign tumors if it is performed in specialized departments of hepatobiliary surgery. The benefit of surgery is significantly associated with preoperative symptoms of patients with FNH.
This case reports also demonstrates that although imaging modalities and liver biopsy have important advantages in the diagnosis of benign and malignant tumors of the liver, misdiagnosis or missed diagnosis would occur, and a definitive diagnostic method for hepatic tumors remains to be established, especially when the tumor is large or with multiple lesions. And surgical resection should only be indicated in patients with tumor-specific symptoms.
Acknowledgments
The authors would like to thank the Department of Pathology of Xijing Hospital for the histopathological examination.
Funding: This study was supported by National Natural Science Foundation of China (grant No. 81572916 to Guo-Qiang Bao).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/dmr-20-157
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-20-157). Both authors report National Natural Science Foundation of China (No. 81572916) Funding for this study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Erickson LA, Torbenson MS. Hepatic Focal Nodular Hyperplasia. Mayo Clin Proc 2020;95:1557-8. [Crossref] [PubMed]
- Kinoshita M, Takemura S, Tanaka S, et al. Ruptured focal nodular hyperplasia observed during follow-up: a case report. Surg Case Rep 2017;3:44. [Crossref] [PubMed]
- Bioulac-Sage P, Balabaud C, Bedossa P, et al. Pathological diagnosis of liver cell adenoma and focal nodular hyperplasia: Bordeaux update. J Hepatol 2007;46:521-7. [Crossref] [PubMed]
- Handra-Luca A, Paradis V, Vilgrain V, et al. Multiple mixed adenoma-focal nodular hyperplasia of the liver associated with spontaneous intrahepatic porto-systemic shunt: a new type of vascular malformation associated with the multiple focal nodular hyperplasia syndrome? Histopathology 2006;48:309-11. [Crossref] [PubMed]
- Hayano S, Naganuma A, Okano Y, et al. A case of idiopathic portal hypertension associated with nodular regenerative hyperplasia-like nodule of the liver and mixed connective tissue disease. Nihon Shokakibyo Gakkai Zasshi 2016;113:828-36. [PubMed]
- Ogawa A, Ogawa E, Yamamoto S, et al. Case of glycogen storage disease type VI (phosphorylase deficiency) complicated by focal nodular hyperplasia. Pediatr Int 2010;52:e150-3. [Crossref] [PubMed]
- Nat L, Poanta LI. Focal nodular hyperplasia (FNH). Rom J Intern Med 2014;52:45-9. [PubMed]
- Libbrecht L, Cassiman D, Verslype C, et al. Clinicopathological features of focal nodular hyperplasia-like nodules in 130 cirrhotic explant livers. Am J Gastroenterol 2006;101:2341-6. [Crossref] [PubMed]
- Li T, Qin LX, Ji Y, et al. Atypical hepatic focal nodular hyperplasia presenting as acute abdomen and misdiagnosed as hepatocellular carcinoma. Hepatol Res 2007;37:1100-5. [Crossref] [PubMed]
- Amisaki M, Honjo S, Iida N, et al. Focal nodular hyperplasia that mimicked a liver metastasis from a soft tissue sarcoma: a case report. Surg Case Rep 2017;3:59. [Crossref] [PubMed]
- Herman P, Pugliese V, Machado MA, et al. Hepatic adenoma and focal nodular hyperplasia: differential diagnosis and treatment. World J Surg 2000;24:372-6. [Crossref] [PubMed]
- Bode AM, Dong Z, Wang H. Cancer prevention and control: alarming challenges in China. Natl Sci Rev 2016;3:117-27. [Crossref] [PubMed]
- Taimr P, Broker MEE, Dwarkasing RS, et al. A Model-Based Prediction of the Probability of Hepatocellular Adenoma and Focal Nodular Hyperplasia Based on Characteristics on Contrast-Enhanced Ultrasound. Ultrasound Med Biol 2017;43:2144-50. [Crossref] [PubMed]
- Wells ML, Hough DM, Fidler JL, et al. Benign nodules in post-Fontan livers can show imaging features considered diagnostic for hepatocellular carcinoma. Abdom Radiol (NY) 2017;42:2623-31. [Crossref] [PubMed]
- Grieser C, Steffen IG, Kramme IB, et al. Gadoxetic acid enhanced MRI for differentiation of FNH and HCA: a single centre experience. Eur Radiol 2014;24:1339-48. [Crossref] [PubMed]
- Yoneda N, Matsui O, Kitao A, et al. Hepatocyte transporter expression in FNH and FNH-like nodule: correlation with signal intensity on gadoxetic acid enhanced magnetic resonance images. Jpn J Radiol 2012;30:499-508. [Crossref] [PubMed]
- Guo Y, Li W, Cai W, et al. Diagnostic Value of Gadoxetic Acid-Enhanced MR Imaging to Distinguish HCA and Its Subtype from FNH: A Systematic Review. Int J Med Sci 2017;14:668-74. [Crossref] [PubMed]
- Newerla C, Schaeffer F, Terracciano L, et al. Multiple FNH-Like Lesions in a Patient with Chronic Budd-Chiari Syndrome: Gd-EOB-Enhanced MRI and BR1 CEUS Findings. Case Rep Radiol 2012;2012:685486 [Crossref] [PubMed]
- Choi JY, Lee HC, Yim JH, et al. Focal nodular hyperplasia or focal nodular hyperplasia-like lesions of the liver: a special emphasis on diagnosis. J Gastroenterol Hepatol 2011;26:1004-9. [Crossref] [PubMed]
- Frohlich JM. MRI of focal nodular hyperplasia (FNH) with gadobenate dimeglumine (Gd-BOPTA) and SPIO (ferumoxides): an intra-individual comparison. J Magn Reson Imaging 2004;19:375-6; author reply 376. [PubMed]
- Grazioli L, Morana G, Kirchin MA, et al. MRI of focal nodular hyperplasia (FNH) with gadobenate dimeglumine (Gd-BOPTA) and SPIO (ferumoxides): an intra-individual comparison. J Magn Reson Imaging 2003;17:593-602. [Crossref] [PubMed]
- Tselikas L, Pigneur F, Roux M, et al. Impact of hepatobiliary phase liver MRI versus Contrast-Enhanced Ultrasound after an inconclusive extracellular gadolinium-based contrast-enhanced MRI for the diagnosis of benign hepatocellular tumors. Abdom Radiol (NY) 2017;42:825-32. [Crossref] [PubMed]
- Aznar DL, Ojeda R, Garcia EU, et al. Focal nodular hyperplasia (FNH): a potential cause of false-positive positron emission tomography. Clin Nucl Med 2005;30:636-7. [Crossref] [PubMed]
- Deniz K, Moreira RK, Yeh MM, et al. Steatohepatitis-like Changes in Focal Nodular Hyperplasia, A Finding to Distinguish From Steatohepatitic Variant of Hepatocellular Carcinoma. Am J Surg Pathol 2017;41:277-81. [Crossref] [PubMed]
- Schmitz D, Kohler HH, Heussel CP, et al. Lymphoma-simulating presentation of focal nodular hyperplasia (FNH) of the liver. Z Gastroenterol 2001;39:219-24. [Crossref] [PubMed]
- Li AJ, Zhou WP, Wu MC. Diagnosis and treatment of hepatic focal nodular hyperplasia: report of 114 cases. Zhonghua Wai Ke Za Zhi 2006;44:321-3. [PubMed]
- Yan JY, Wang MQ, Liu FY, et al. Super selective transcatheter arterial embolization for treatment of focal nodular hyperplasia of the liver: report of 21 cases. Zhonghua Yi Xue Za Zhi 2012;92:2893-6. [PubMed]
- Huang D, Chen Y, Zeng Q, et al. Transarterial embolization using pingyangmycin lipiodol emulsion and polyvinyl alcohol for the treatment of focal nodular hyperplasia of the liver. Hepatogastroenterology 2011;58:1736-41. [Crossref] [PubMed]
- Zhang JW, Wang CF, Liu Q, et al. Diagnosis and treatment of focal nodular hyperplasia of liver: report of 23 cases. Zhonghua Yi Xue Za Zhi 2007;87:2531-3. [PubMed]
- Perrakis A, Demir R, Muller V, et al. Management of the focal nodular hyperplasia of the liver: evaluation of the surgical treatment comparing with observation only. Am J Surg 2012;204:689-96. [Crossref] [PubMed]
- Navarro AP, Gomez D, Lamb CM, et al. Focal nodular hyperplasia: a review of current indications for and outcomes of hepatic resection. HPB (Oxford) 2014;16:503-11. [Crossref] [PubMed]
- Birn J, Williams TR, Croteau D, et al. Transarterial embolization of symptomatic focal nodular hyperplasia. J Vasc Interv Radiol 2013;24:1647-55. [Crossref] [PubMed]
- Hussain SM, Terkivatan T, Zondervan PE, et al. Focal nodular hyperplasia: findings at state-of-the-art MR imaging, US, CT, and pathologic analysis. Radiographics 2004;24:3-17; discussion 18-9. [Crossref] [PubMed]
- Nguyen BN, Flejou JF, Terris B, et al. Focal nodular hyperplasia of the liver: a comprehensive pathologic study of 305 lesions and recognition of new histologic forms. Am J Surg Pathol 1999;23:1441-54. [Crossref] [PubMed]
- Happaerts S, Foucault A, Billiard JS, et al. Combined hepatocellular-cholangiocarcinoma in a patient with Abernethy malformation and tetralogy of Fallot A case report. Hepatology 2016;64:1800-2. [PubMed]
- Mistinova J, Valacsai F, Varga I. Congenital absence of the portal vein--Case report and a review of literature. Clin Anat 2010;23:750-8. [Crossref] [PubMed]
- Perrakis A, Vassos N, Grutzmann R, et al. What is Changing in Indications and Treatment of Focal Nodular Hyperplasia of the Liver. Is There Any Place for Surgery? Ann Hepatol 2017;16:333-41. [Crossref] [PubMed]
Cite this article as: Yang ZY, Bao GQ. Focal nodular hyperplasia misdiagnosed as hepatocellular carcinoma: a case report and literature review. Dig Med Res 2021;4:17.


