
Surgical management of intrahepatic cholangiocarcinoma: a narrative review
Introduction
Cholangiocarcinoma (CCA) is the second most common primary liver malignancy after hepatocellular carcinoma (HCC) and accounts for approximately 15% of all primary liver cancers (1). The tumor cells can originate from the epithelium of any segment of the whole biliary tree while HCC originates from the hepatocytes in liver (2). Anatomically, it can be divided into intrahepatic CCA (iCCA), perihilar CCA (pCCA) and distal CCA (dCCA) according to the latest American Joint Committee on Cancer (AJCC) staging system (3). iCCA refers to a tumor arising from the intrahepatic bile ducts in the periphery of the liver proximal to the second-order bile ducts. pCCA refers to a tumor arising from the bile duct between the bifurcation of the right and left hepatic ducts and the confluence of the cystic duct joining the common hepatic duct. dCCA refers to a tumor arising from the common bile duct distal to the union of the cystic duct and the common hepatic duct. iCCA can be further subdivided into the following four types according to its gross appearance: (I) the mass-forming type: present as a localized tumor with a radial growth pattern and a demarcative border in the liver parenchyma; (II) the periductal-infiltrating type: present as a diffuse and ill-defined growth longitudinally along the intrahepatic bile ducts; (III) the intraductal growth type: present as a papillary or a polypoid lesion in the lumen of the bile duct; and (IV) the mixed type: present as a tumor consisting of features of more than one of the above three types simultaneously. The clinical characteristics and biological behaviors may also differ among different types of iCCA (4).
Although the prevalence of iCCA is rare, there has been a global trend toward increasing incidence over the past few decades (5). The risk of developing iCCA may be related to primary sclerosing cholangitis (PSC), parasite infection with hepatobiliary flukes (Opisthorchis viverrini and Clonorchis sinensis), chronic viral hepatitis B or C, liver cirrhosis, hepatolithiasis, metabolic syndrome, nonalcoholic fatty liver disease, congenital biliary tract malformations, smoking, alcohol consumption, and environmental toxic substances (5,6). However, iCCA can also develop in a certain number of patients without any evidence of exposure to the identified risk factors. Once iCCA is diagnosed, surgical resection is the most effective treatment option for cure. Unfortunately, only a minority of patients are eligible for surgery. In patients with unresectable advanced or metastatic diseases, systemic chemotherapy offers unsatisfactory results, with a median overall survival (OS) of 11.7 months (7).
The aim of this paper is to review the recent advances in knowledge of surgical management of iCCA, including pre-operative assessment and preparation, surgical approaches and lymphadenectomy, prognosis and important prognostic factors, minimally invasive surgery, adjuvant and neoadjuvant therapies, and liver transplantation, and indicate the potential future research direction in these fields. The review of the scientific literature was performed by searching PubMed database for articles published from year 2000 through January 2021. The search strategy for PubMed was by using search string: (intrahepatic cholangiocarcinoma[Title]) AND (liver resection[Title] or transplantation[Title] or hepatectomy[Title] or surgery[Title] or neoadjuvant[Title] or adjuvant[Title]). Only articles published in English language were included in the review study and case reports were excluded. References of selected articles were also reviewed to identify any missed studies. We present the following article in accordance with the narrative review checklist (available at http://dx.doi.org/10.21037/dmr-21-14).
Preoperative evaluation and management
Preoperative image assessment has two main objectives: (I) to ensure a safe resection with a negative surgical margin (R0) which is defined as no microscopic residual tumor cell on the cut surface of surgical specimen by pathology examination (8), and (II) to select oncologically feasible patients who can benefit from surgery. It is essential to define the extent of tumor involvement and identify possible extrahepatic and lymph node metastases prior to surgical planning. A high-quality triple-phase CT scan is mandatory for initial diagnosis and staging. Typically, the CT image finding of iCCA is a hypoattenuated mass with irregular peripheral enhancement in the arterial phase and gradual filling enhancement toward the central part of the tumor in the delayed phase (9). In most patients, tumor size, tumor number, tumor location, major vascular invasion, biliary tract involvement, lymph node metastases, extrahepatic diseases, and peritoneal seeding can be assessed with a CT scan (Figure 1). Additional chest CT scan and whole-body bone scan should be considered in high-risk patients with large tumors, multifocal intrahepatic lesions, lymphadenopathy, suspicious extrahepatic tumors, and high serum levels of carcinoembryonic antigen (CEA) or carbohydrate antigen 19-9 (CA19-9). According to recent studies, hepatobiliary phase images of gadolinium ethoxybenzyl diethylenetriamine penta-acetic acid (Gd-EOB-DTPA)-enhanced MRI can enhance the conspicuity of iCCA lesions and provide better ability to identify daughter nodules and intrahepatic metastases. Therefore, it can be helpful in surgical planning, especially in patients with multiple liver tumors (10,11). FDG PET/CT has been shown to be beneficial to the detection of occult metastases and can change the strategy of surgical management. Caution should be taken when interpreting negative findings as there is a risk of false-negative results (12,13).
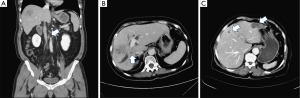
A sufficient functional remnant liver volume (FRLV) after hepatectomy must be guaranteed by 3D-CT volumetry in the preoperative planning (14), especially when extended major hepatectomy is anticipated due to tumors with large size, central location, major vascular invasion, or bile duct involvement. The FRLV is calculated according to the formula: [(total liver volume − resected volume)/(total liver volume − tumor volume)] ×100% (15). In patients with preserved liver function and no evidence of underlying liver disease, an FRLV >30% is acceptable (16). If the FRLV is insufficient, preoperative portal vein embolization can be utilized to increase the FRLV and avoid liver failure after hepatectomy (17). In patients with chronic liver disease or cirrhotic changes, the future FRLV should be at least 40%, and remnant liver function should be assessed by other liver function tests, such as the indocyanine green clearance rate at 15 minutes test, to evaluate the safety of major resection (18). In patients with obstructive jaundice, preoperative biliary drainage should be considered in those with concomitant cholangitis or a small future FRLV as these factors may be associated with surgical mortality after hepatectomy (19).
Principle of surgical approach and prognosis
The principle of the surgical approach to iCCA includes liver resection to obtain a negative surgical margin and regional lymphadenectomy of the hepatic hilum. Multifocal liver disease, lymph node metastasis status, and distant metastasis should be determined during the initial surgical exploration. Multiple intrahepatic tumors can represent a metastatic disease status and should be considered a relative contraindication to resection, except in highly selected patients with limited multifocal disease. Lymph node metastases beyond the hepatic hilum can be treated as distant metastases and surgery is contraindicated (20). A poor prognosis is expected in patients with gross lymph node metastases to the hepatic hilum, and liver resection should be selectively performed (21,22). In a curative operation attempting to obtain negative margin, the R0 resection rate was between 58.7% and 87.6% (23-29) and varied depending on the experience of the surgical team, cancer stage, and gross type of iCCA (26,27).
More than half of the patients develop a recurrence after resection of iCCA (29-31). The median disease-free survival (DFS) in various large cohort studies was between 10 and 23 months (25,32,33). Most recurrences occur within the first 2 years after primary resection, and the most frequent site of recurrence is the intrahepatic region (25,34). Extrahepatic recurrence can develop in 40% to 50% of patients with recurrent disease, with half of the extrahepatic recurrences occurring at the same time as intrahepatic relapse (32). Even after 2 years of DFS, a relapse can develop, and distant metastases become the dominant pattern (25). Tumor recurrence is a major prognostic factor for patient survival, and most patients die from recurring diseases. In the literature, the median OS of surgical patients was between 14.1 and 47.8 months, and the 5-year OS rate was between 15.1% and 45.6% (24-27,30,32,33,35-41). The detailed survival results of the recent large series are shown in Table 1.
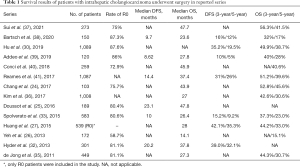
Full table
Surgical margin
The patient’s postoperative survival is strongly influenced by several important prognostic factors, including tumor size, tumor number, vascular invasion, lymph node metastasis, surgical margin status, tumor cell differentiation, preoperative serum CEA level, hepatolithiasis, and gross tumor pattern (24-31,33-35,42-44). Achieving an R0 resection is believed to have a large impact on a patient’s long-term survival, and the 5-year OS rate with a positive surgical margin has been reported to be zero (29). In a multi-institutional study of 583 patients, 95 (16.3%) patients with R1 resection had poorer long-term survival than patients with R0 resection, and there was an increasing trend toward worsening DFS and OS as the distance of surgical margin decreased (33). Although the results of several studies suggested that the width of surgical margin should be at least 5 to 10 mm (33,38), other studies reported conflicting results (45,46). A retrospective study of 635 patients who underwent R0 resection, which analyzed the data from the Japan nationwide survey of iCCA, found that the width of surgical margin appeared to have limited survival benefits to patients except those without lymph node metastasis (47). Similar results were also obtained in the study by Farges et al. (45); R1 resection was the worst independent prognostic factor in patients with N0 disease, and a surgical margin of at least 5 mm was suggested. However, the influence of the surgical margins on survival diminished in the group of patients with lymph node metastases. Collectively, these results suggest that the impact of the surgical margin status on survival can be overwhelmed by the presence of lymph node metastases. Therefore, according to current practice guidelines, the goal of surgery is to achieve a negative margin, regardless of the length of the surgical margin (20).
Lymph node metastasis and Lymphadenectomy
Lymph node metastases has been proven to be the most important prognostic factor in several studies, and the presence of lymph node metastases can reduce the prognostic impact of other pathologic factors, such as tumor size, positive margins, multiple tumors, and vascular invasion (35,42,44,47-49). In a study of 449 iCCA surgical patients reported by de Jong et al. (35), among patients with no lymph node metastasis, multiple tumors and vascular invasion were worse strong prognostic factors associated with worse survival. On the contrary, among patients with lymph node metastasis, tumor number and vascular invasion became unable to stratify patients into different prognostic groups, and lymph node metastasis became the only prognostic factor. Although there is still controversy as to whether routine lymph node dissection actually provides therapeutic benefits and prolongs patient survival (42,48,50), the consensus in current practice is in favor of lymphadenectomy for accurate staging information, prognosis prediction, and adjuvant strategy development (20-22). However, in recent large cohort studies, the percentage of patients who had lymph node dissection remained low, ranging from 44.6% to 78.4% (29,35,48,51). The reasons for an incomplete lymphadenectomy could be a preoperative diagnosis of HCC, insignificant lymphadenopathy on preoperative images, advanced disease unable to complete curative resection, or intolerance to extensive dissection due to the patient’s condition. Since the presence of lymph node metastases is associated with a late stage tumor, multiple tumors, vascular invasion, and high preoperative serum CEA level, it has been suggested that lymph node dissection could be omitted in patients with small, solitary, and peripheral iCCA (24,29,35,52). It should be noted, however, that even in preoperatively radiological nodal-negative patients, the percentage of lymph node metastases can be as 17% to 45% (50,52). The quality of the lymph node dissection is also important for optimal tumor staging. The latest 8th edition of the AJCC Staging System recommends harvesting at least six lymph nodes (3). In a large multicenter cohort study of 1,154 patients by Bagante et al. (51), only one-fourth of the patients had a sufficient number of harvested lymph nodes for accurate staging. The study also found that the number of harvested lymph nodes is related to the prognosis of patients with N0 disease, which may imply the importance of a qualified lymphadenectomy for precise tumor staging.
Multifocal tumors
The multinodularity of tumors has been recognized as an important prognostic factor and may reflect the aggressive biological nature of the disease in terms of associations with a high rate of lymph node metastases, vascular invasions, extrahepatic involvement, and poor differentiation (29,39,43,53). In a recent study of 1,013 iCCA patients after resection, including 821 patients with solitary tumors, 185 patients with multiple tumors, and 27 patients with oligoextrahepatic metastases, Buettner et al. (43) discovered that the presence of two liver tumors did not preclude patients from long-term survival with a 5-year OS rate of 28%. On the other hand, the existence of three or more intrahepatic lesions was an independent poor prognostic factor for the OS. Notably, patients with three or more tumors had similar survival results (median OS 15.3 months and 5-year OS rate 8.6%) to patients with oligoextrahepatic metastases (median OS 14.9 months and 5-year OS rate 10.6%). Theoretically, multifocal or bilobular disease could be considered a metastatic disease, and surgery could offer limited additional benefit in comparison with other locoregional treatment modalities (54). Therefore, according to the current consensus, surgery can only be considered in patients with limited multifocal disease. However, the prognostic impact of tumor distribution patterns such as peri-tumor satellites, multicentric tumors, and bilobular disease remains controversial, and several studies have demonstrated conflicting results (39,40). To determine whether patients with multiple tumors can benefit from surgery and decide the final treatment strategy, other important concomitant prognostic factors such as lymph node metastases, vascular invasion, and tumor cell differentiation should be considered simultaneously by the surgical team (39,53).
Vascular invasion
Patients with vascular invasion are more likely to have advanced disease and poorer survival outcomes than patients without vascular invasion (25,30). In contrast to HCC, in which a major vascular invasion was recognized as an important prognostic factor and integrated into the current AJCC staging system (55), the true prognostic influence of a major vascular invasion is still undetermined and has therefore not been included in the current AJCC staging system for iCCA. Hu et al. (30) analyzed the survival results of 1,089 patients, including 149 patients with macrovascular invasion, and found that macrovascular invasion was associated with inferior DFS and OS. However, as long as a curative resection could be performed, patients who had undergone a major vascular resection showed similar short-term perioperative outcomes in terms of surgical morbidity and mortality and long-term oncological survival results as patients who had not undergone a major vascular resection (41,56). Therefore, according to the current consensus, extensive surgery requiring major vascular resection is not a contraindication to curative surgery.
Staging and nomograms
Although iCCA is the second most common primary liver malignancy, its incidence is rare, and limited understanding of the disease has meant that the value of prognostic factors has been uncertain for the past several decades. Initially, in the 6th edition of the AJCC TNM staging system for iCCA, the classification was the same as that of HCC based on the data exclusively referring from HCC. In addition, several other staging systems such as the LCSGJ, the Nathan, and the Okabayashi have been proposed, but none of these staging systems have been able to provide a satisfactory ability for stage stratification (57,58). An independent staging system for iCCA was adapted in the 7th edition of the AJCC staging system, and an improved ability to discriminate patient survival by stage was noted (59). The staging system was recently revised to the 8th edition (3) by (I) reclassifying T1 solitary tumors without vascular invasion into T1a and T1b according to a tumor size greater than 5 cm; (II) combining T2a solitary tumors with vascular invasion and T2b multiple tumors in a newly incorporated T2 stage; (III) reclassifying tumors involving local extrahepatic structures by direct invasion from the previous T3 stage to the T4 stage; (VI) omission of tumors with periductal invasion in the previous T4 stage; and (V) reclassifying N1 disease with no distant metastasis into stage IIIB instead of stage IVA. However, these modifications did not significantly improve the prognostic discrimination compared to the previous 7th edition, especially in patients with stage T3 disease (36,60,61). Therefore, an ideal staging system for prognostic stratification in surgical patients remains to be investigated. In addition to the imprecise classification at the T stage, the inability to reflect the biological aggressiveness of the tumor by just estimating anatomical/pathological features may be another reason for the incomplete performance of the current staging system, and the inclusion of preoperative serum CEA level, CA19-9 level, and tumor cell differentiation has been suggested to improve the predictive power (58,62). In order to predict the prognosis of the individual patient more precisely, several prognostic nomograms have been proposed (63-65). Hyder et al. (65) performed an international multicenter study of 514 patients in 13 major Eastern and Western hepatobiliary centers and developed a nomogram based on 6 independent prognostic markers: patient age, tumor size, tumor number, lymph node status, vascular invasion, and underlying cirrhosis. The nomogram showed better ability of survival prediction than the conventional AJCC staging system (65). Serum tumor marker values have also been incorporated into the other two nomogram systems by Yeh et al. (63) and Wang et al. (64). The superiority of the prediction of survival was also demonstrated in these studies in validation cohorts. (64,65).
Re-resection for recurrence
Unfortunately, even after curative resection for iCCA, a large proportion of patients developed recurrence within 5 years of surgery, and recurrent disease ultimately led to mortality in most patients (28,66-69). Treatment options for recurrent tumors include repeat resection, local ablation, intra-arterial therapy, selective internal radiation therapy (SIRT), systemic chemotherapy, and best supportive care. More than half of the recurrences developed only intrahepatically, and it was assumed that resection of the recurrent tumor could offer a survival advantage (28,66,69). In previous studies, the OS of recurrent patients who had surgical resection was better than that of recurrent patients who received other treatment modalities. (28,69). Furthermore, after successful resection of recurrent iCCA, the OS of patients was similar to that of non-recurrent patients after the first operation. However, due to the selection bias from retrospective studies, care should be taken to interpret these results. In a study of 400 patients with recurrent iCCA by Spolverato et al. (67), repeated liver resection offered only a modest survival benefit with a median time to second recurrence of 11.5 months. The selection of patients who will really benefit from repeated operation should be thoughtful. Multiple recurrent nodules, the size of the recurrent tumor, time to recurrence, lymph node status, liver cirrhosis, and the number of primary tumors are independent risk factors for survival and should be considered selection criteria (28,66,68). As a result, repeated resection can only to be applied to a minority of patients. In several cohort studies, the resection rate for recurrences was between 9% and 28.6% (28,66-69).
Minimally invasive approach
Although it has been well known that the use of laparoscopic liver resection in HCC patients can improve perioperative outcomes without compromising long-term oncological survival (70), the reported series of laparoscopic resection in iCCA patients are rare, which may reflect the complexity of the disease nature that often requires extensive resection and lymph node dissection (71). From the few reports available comparing laparoscopic with open approaches to iCCA, less blood loss, less blood transfusion requirement, shorter hospital stays, and faster functional recovery were observed in the laparoscopic group, but the R0 resection rate, the recurrence-free survival, and the OS were observed similar (72-76). However, previous studies were limited to few laparoscopic case numbers, and the largest number of cases was only 20 (75). Therefore, it should be carefully considered whether these results can be applied to all iCCA patients. In a larger study of 2,309 iCCA patients who underwent surgery, the data from the National Cancer Database of the United States were retrieved and analyzed (77). The rate of lymph node dissection was significantly lower in patients with laparoscopic liver resection (39%) than in patients with open liver resection (61%). In addition, of the 120 patients who had both laparoscopic liver resection and lymph node dissection performed, up to 31% of patients had only one lymph node harvested. These results may suggest that performing the laparoscopic resection in iCCA patients harbors the risk of inadequate nodal staging. The advantage of robotic-assisted surgery is that it enables delicate and precise dissection in a confined space and can facilitate hepatic hilum lymph node dissection (78). In recent studies, increasing evidence has gradually demonstrated that robotic surgery has similar surgical-oncological effectiveness to open and traditional laparoscopic surgeries in the treatment of iCCA (79-81). In general practice, a minimally invasive approach to iCCA may be feasible in selected patients with peripheral and isolated tumors (71). In addition, the initial laparoscopic exploration could also be considered in high-risk patients with advanced disease. Presumably, almost one-third of patients with hepatobiliary cancer could benefit from avoiding unnecessary laparotomy by staging laparoscopy according to the literature (82-84).
Adjuvant therapy
Although surgery is the primary option of curative therapy, the incidence of disease relapse remains high, especially in advanced stages. There is an urgent need to develop effective adjuvant therapy to reduce the rate of recurrence and extend patient survival. However, the current recommendations for adjuvant therapy are mainly based on several retrospective studies, consensuses, and very few clinical trial results. A retrospective study analyzed the data of 638 surgical patients from the United States National Cancer Database and found that patients with positive lymph nodes or positive margins could benefit from adjuvant chemotherapy or chemoradiotherapy (85). The benefits of adjuvant chemotherapy in high-risk patients (i.e., patients with a large tumor size, multiple tumors, vascular invasion, advanced T stages, or the periductal-infiltrating type) were also observed in two multi-institutional researches and one matched-pair analysis (28,86,87). Gemcitabine-based chemotherapy were the most favorable regimens and comprised more than half of the treatment protocols in these studies. A meta-analysis of 15 retrospective series and 5,060 patients showed a significant advantage of adjuvant chemotherapy for patient survival (hazard ratio 0.66, 95% CI: 0.55–0.79, P<0.001) (88). The intravenous route of chemotherapy injection and the administration of a gemcitabine-based regimen were associated with improved OS in the study. However, no improvement of DFS was observed in the subgroup analysis.
The current standard treatment for unresectable iCCA is systemic chemotherapy with cisplatin plus gemcitabine according to the results of the ABC-02 trial (7). However, the efficacy of this regimen in the adjuvant setting for iCCA seems difficult to evaluate in prospective randomized trials due to the limited number of clinical trials and the heterogeneity of target patients, who usually include not only those with iCCA, but also those with extrahepatic CCA, gallbladder cancer, or periampullary carcinoma (89-92). The PRODGE 12-ACCORD 18 (UNICANCER GI) study was a randomized phase III trial in which the adjuvant therapy with gemcitabine plus oxaliplatin (GEMOX) was compared with the observation in patients with resected biliary tract cancer (89). There were 194 patients in the study: 86 with iCCA, 15 with pCCA, 55 with dCCA, and 17 with gallbladder cancer. No survival benefit was observed in the treatment group compared to the control observation group. The subgroup analysis showed no survival benefit of the adjuvant GEMOX in patients with iCCA. In the phase III Bile Duct Cancer Adjuvant Trial (BCAT) in Japan, the efficacy of the adjuvant gemcitabine was compared with the observation in patients with resected bile duct cancer, but mainly patients with pCCA and dCCA were included (90). There was also no significant difference in survival between the treatment group and the observation group. The recently conducted BICAP phase III trial revealed a potentially survival benefit of adjuvant therapy with capecitabine in patients with resected biliary tract cancer (91). A total of 447 patients were enrolled, and 223 patients, including 43 with iCCA, were randomly assigned to the capecitabine group; 224 patients, including 41 with iCCA, were randomly assigned to the observation group. Although the study showed no survival benefit in the intention-to-treat analysis, with a median survival in the treatment group of 51.1 months compared to 36.4 months in the observation group (hazard ratio 0.81, 95% CI: 0.63–1.04, P=0.097), improved OS was still observed in the per-protocol analysis, with a median survival of 53 months versus 36 months (hazard ratio 0.75, 95% CI: 0.58–0.97; P=0.028). Notably, no survival benefit was observed in the subgroup analysis of iCCA. Based on these evidences, in the updated practice guidelines, the preferred treatment regimen of adjuvant therapy is capecitabine for a period of 6 months after surgery (20,93). The ACTICCA-1 trial (NCT 02170090) is currently ongoing and recruiting patients (94). In relation to the results of the ABC-02 trial, postoperative patients with CCA and muscle invasive gallbladder carcinoma are randomized in this multinational phase III trial to receive either adjuvant therapy with gemcitabine plus cisplatin or only observation. The upcoming results may provide further information on the choice of adjuvant therapy.
Neoadjuvant therapy
Although the tumor response rate to the classic systemic chemotherapy regimen with cisplatin plus gemcitabine could reach 25.5% (7), the strong evidence of a survival benefit in patients who underwent surgery after neoadjuvant chemotherapy was insufficient, and the role of neoadjuvant therapy remains controversial. In a multi-institutional analysis of 1,057 patients, 62 patients who received preoperative chemotherapy, even though they had more diseases in advanced stages, had a similar median OS and DFS to 995 patients without preoperative chemotherapy (95). In another study by Le Roy et al. (96), 39 (53%) of 74 patients with locally advanced iCCA received liver resection after a median of six cycles of chemotherapy. The median survival of these 39 patients was 24.1 months, which was comparable to the 25.7 months of patients with initially resectable iCCA. SIRT is also seen as another potential method for downstaging (97). In a French study of 137 iCCA patients who underwent upfront surgery and 32 patients who received downstaging treatment (13 chemotherapy and 19 SIRT) for an initially unresectable disease, SIRT was identified as an independent prognostic factor associated with a survival benefit (98). The combination of SIRT and systemic chemotherapy could be a promising strategy to downstage unresectable disease to surgical treatment. In a study of 45 patients treated with SIRT plus chemotherapy for unresectable iCCA, eight patients underwent surgery after successfully downstaged and all patients achieved an R0-resection (99). More recently, the results of a phase II trial on radioembolization plus chemotherapy for the first-line treatment of locally advanced iCCA showed that 22% of patients could be downstaged to surgery (100). In the study, the median recurrence-free survival of surgical patients was not reached after a median follow-up interval of 46 months. Though current evidence may not be sufficient to support routine neoadjuvant therapy in iCCA, limited but promising results from recent studies (e.g., systemic chemotherapy plus SIRT in advanced iCCA) may warrant further researches and clinical trials to investigate the potential role of neoadjuvant therapy in patients with advanced iCCA.
Liver transplantation
While liver transplantation is viewed as an effective method for curative treatment in HCC patients with tumors within the Milan (101) or UCSF (102) criteria, liver transplantation for iCCA has traditionally been considered a contraindication (103,104) due to disappointing results from early series. In 1997, Casavilla et al. reported a case series of 20 patients, the 5-year OS and DFS rates were 18% and 31%, respectively (105). The earlier Canadian experience of 10 patients reported by Ghali et al. revealed a 30% 3-year OS rate after liver transplantation (106). Similar results were also observed from the other studies in which the majority of patients had tumor recurrence within 3 years of liver transplantation and the 5-year survival rate was no more than 38% (107-109). The low survival and high recurrence rates were far behind the results of liver transplantation for non-tumor cirrhotic patients or HCC patients within Milan criteria and discouraged surgeons from using liver transplantation as a treatment option for iCCA (109,110). However, in a recent study, a subgroup of cirrhotic patients with “very early” iCCA, defined as solitary tumor ≤2 cm, achieved an excellent 5-year survival rate of up to 73% after liver transplantation (111). The 5-year tumor recurrence rate of these “very early” iCCAs could be only 18%, compared to 65% of the “advanced” iCCAs which consist of multiple tumors or tumors larger than 2 cm (112). Therefore, patients with a single iCCA smaller than 2 cm should not be precluded as candidates for liver transplantation, as the long-term outcome may be comparable to that of patients with HCC (111-113).
Given the promising results of liver transplantation for unresectable pCCA from a multicenter study in which 214 patients received neoadjuvant therapy followed by liver transplantation and the 5-year recurrence-free survival rate reached 65% (114), liver transplantation for locally advanced iCCA with no evidence of lymph node or distant metastasis has been suggested by experts (115-118). To define which patients could benefit from liver transplantation, a retrospective study by Hong et al. (116) reviewed the data of 40 patients who had liver transplantation for locally advanced iCCA and pCCA. Seven predictive factors for tumor recurrence, namely, multifocal tumors, perineural invasion, an infiltrative growth pattern, a lack of neoadjuvant and adjuvant therapy, a history of PSC, hilum tumors, and lymphovascular invasion, were identified and assigned risk score points. Patients in the low-risk group according to the summed risk score point stratification system could achieve a high 5-year tumor recurrence-free survival rate of up to 78%. Liver transplantation seems to offer better tumor recurrence-free survival than radical liver resection combined with bile duct resection in locally advanced iCCA (117). In particular, the survival benefit was more obvious in transplant patients who received neoadjuvant and adjuvant therapy than in patients with no therapy (5-year tumor recurrence-free survival: 47% versus 20%, P=0.03). In a recent prospective case series study by the Methodist-MD Anderson Joint Cholangiocarcinoma Collaborative Committee (MMAJCCC) (115), six patients with unresectable locally advanced iCCA received liver transplantation under the condition of at least 6 months of radiologically stable or regression disease on neoadjuvant chemotherapy. The median follow-up time for transplant patients was 36 months, and the 5-year OS and DFS rates were 83.3% and 50%, respectively. The author pointed out that chemoresponsiveness can serve as an important surrogate marker of tumor biology behavior in the selection of iCCA patients suitable for liver transplantation. In this series, 5 recipients underwent next-generation sequencing of tumors. KRAS and BAP1 mutations, presumably may be associated with an aggressive phenotype in iCCA, were each identified in different one patient and both patients developed disease relapse. Although these results may not sufficiently conclude the relationship between tumor recurrence and genetic profile, the author believed that tumor genomic profiling would play an important role in identifying patients with a favorable biology for transplantation in the future (115,119).
Conclusions
Surgery is the only treatment that can provide long-term survival for patients with iCCA. Unfortunately, the majority of patients are diagnosed at an advanced stage and the prognosis is compromised by a high recurrence rate even after curative resection. It is important to undergo optimal preoperative imaging studies to identify subtle intrahepatic and extrahepatic lesions and to select suitable surgical candidates. Although large tumor size, multifocal disease, major vascular invasion, and large bile duct involvement do not preclude patients from surgery, a negative surgical margin with sufficient remnant liver function must be ensured when patients receive liver resection. Hepatic hilum lymphadenectomy with an adequate number of harvested lymph nodes during surgery should be a routine practice for accurate disease staging. Postoperative adjuvant chemotherapy could be offered to patients to improve survival, especially in patients with advanced-stage disease. Liver transplantation could provide a survival benefit for patients with a small and single tumor who cannot receive a resection due to liver cirrhosis. Patients with unresectable locally advanced disease who are chemoresponsive and have stable disease after initial neoadjuvant chemotherapy may also be eligible to liver transplantation. However, the selection of patients for liver transplantation may warrant further study. In the future, molecular and genomic profiling information could provide important guidance for surgical strategy decisions.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Yi-Jen Chen) for the series “Locoregional and systemic treatment in intrahepatic cholangiocarcinoma” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the narrative review checklist. Available at http://dx.doi.org/10.21037/dmr-21-14
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-21-14). The series “Locoregional and systemic treatment in intrahepatic cholangiocarcinoma” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017;24:1073274817729245 [Crossref] [PubMed]
- International Agency for Research on Cancer. WHO classification of tumours of the digestive system. Lyon: International Agency for Research on Cancer, 2019.
- Amin MB, Edge S, Greene F, et al. AJCC cancer staging manual. 8th ed. Switzerland: Springer International Publishing, 2017.
- Vijgen S, Terris B, Rubbia-Brandt L. Pathology of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2017;6:22-34. [Crossref] [PubMed]
- Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13:261-80. [Crossref] [PubMed]
- Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford) 2008;10:77-82. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Wittekind C, Compton C, Quirke P, et al. A uniform residual tumor (R) classification: integration of the R classification and the circumferential margin status. Cancer 2009;115:3483-8. [Crossref] [PubMed]
- Seo N, Kim DY, Choi JY. Cross-sectional imaging of intrahepatic cholangiocarcinoma: Development, growth, spread, and prognosis. AJR Am J Roentgenol 2017;209:W64-75 [Crossref] [PubMed]
- Péporté AR, Sommer WH, Nikolaou K, et al. Imaging features of intrahepatic cholangiocarcinoma in Gd-EOB-DTPA-enhanced MRI. Eur J Radiol 2013;82:e101-6. [Crossref] [PubMed]
- Kang Y, Lee JM, Kim SH, et al. Intrahepatic mass-forming cholangiocarcinoma: enhancement patterns on gadoxetic acid-enhanced MR images. Radiology 2012;264:751-60. [Crossref] [PubMed]
- Sacks A, Peller PJ, Surasi DS, et al. Value of PET/CT in the management of primary hepatobiliary tumors, part 2. AJR Am J Roentgenol 2011;197:W260-5 [Crossref] [PubMed]
- Cillo U, Fondevila C, Donadon M, et al. Surgery for cholangiocarcinoma. Liver Int 2019;39:143-55. [Crossref] [PubMed]
- Bégin A, Martel G, Lapointe R, et al. Accuracy of preoperative automatic measurement of the liver volume by CT-scan combined to a 3D virtual surgical planning software (3DVSP). Surg Endosc 2014;28:3408-12. [Crossref] [PubMed]
- Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 1997;26:1176-81. [PubMed]
- Adam R, Lucidi V, Bismuth H. Hepatic colorectal metastases: methods of improving resectability. Surg Clin North Am 2004;84:659-71. [Crossref] [PubMed]
- Nagino M, Kamiya J, Nishio H, et al. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg 2006;243:364-72. [Crossref] [PubMed]
- Guglielmi A, Ruzzenente A, Conci S, et al. How much remnant is enough in liver resection? Dig Surg 2012;29:6-17. [Crossref] [PubMed]
- Wiggers JK, Groot Koerkamp B, Cieslak KP, et al. Postoperative Mortality after Liver Resection for Perihilar Cholangiocarcinoma: Development of a Risk Score and Importance of Biliary Drainage of the Future Liver Remnant. J Am Coll Surg 2016;223:321-31.e1. [Crossref] [PubMed]
- Benson AB, D’Angelica MI, Abbott DE, et al. NCCN clinical Practive Guideline in Oncology, Hepatobiliary Cancers, Version 5.2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed January 9 2021
- Weber SM, Ribero D, O’Reilly EM, et al. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:669-80. [Crossref] [PubMed]
- Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89. [Crossref] [PubMed]
- Spolverato G, Kim Y, Ejaz A, et al. Conditional Probability of Long-term Survival After Liver Resection for Intrahepatic Cholangiocarcinoma: A Multi-institutional Analysis of 535 Patients. JAMA Surg 2015;150:538-45. [Crossref] [PubMed]
- Chang ME, Lei HJ, Chen MH, et al. Evaluation of prognostic factors and implication of lymph node dissection in intrahepatic cholangiocarcinoma: 10-year experience at a tertiary referral center. J Chin Med Assoc 2017;80:140-6. [Crossref] [PubMed]
- Doussot A, Gonen M, Wiggers JK, et al. Recurrence Patterns and Disease-Free Survival after Resection of Intrahepatic Cholangiocarcinoma: Preoperative and Postoperative Prognostic Models. J Am Coll Surg 2016;223:493-505.e2. [Crossref] [PubMed]
- Yeh CN, Yeh TS, Chen TC, et al. Gross pathological classification of peripheral cholangiocarcinoma determines the efficacy of hepatectomy. J Gastroenterol 2013;48:647-59. [Crossref] [PubMed]
- Hwang S, Lee YJ, Song GW, et al. Prognostic Impact of Tumor Growth Type on 7th AJCC Staging System for Intrahepatic Cholangiocarcinoma: a Single-Center Experience of 659 Cases. J Gastrointest Surg 2015;19:1291-304. [Crossref] [PubMed]
- Yamashita YI, Shirabe K, Beppu T, et al. Surgical management of recurrent intrahepatic cholangiocarcinoma: predictors, adjuvant chemotherapy, and surgical therapy for recurrence: A multi-institutional study by the Kyushu Study Group of Liver Surgery. Ann Gastroenterol Surg 2017;1:136-42. [Crossref] [PubMed]
- Marubashi S, Gotoh K, Takahashi H, et al. Prediction of the postoperative prognosis of intrahepatic cholangiocarcinoma (ICC): importance of preoperatively- determined anatomic invasion level and number of tumors. Dig Dis Sci 2014;59:201-13. [Crossref] [PubMed]
- Hu LS, Weiss M, Popescu I, et al. Impact of microvascular invasion on clinical outcomes after curative-intent resection for intrahepatic cholangiocarcinoma. J Surg Oncol 2019;119:21-9. [Crossref] [PubMed]
- Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84-96. [Crossref] [PubMed]
- Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013;153:811-8. [Crossref] [PubMed]
- Spolverato G, Yakoob MY, Kim Y, et al. The Impact of Surgical Margin Status on Long-Term Outcome After Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2015;22:4020-8. [Crossref] [PubMed]
- Miwa S, Miyagawa S, Kobayashi A, et al. Predictive factors for intrahepatic cholangiocarcinoma recurrence in the liver following surgery. J Gastroenterol 2006;41:893-900. [Crossref] [PubMed]
- de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140-5. [Crossref] [PubMed]
- Kim Y, Moris DP, Zhang XF, et al. Evaluation of the 8th edition American Joint Commission on Cancer (AJCC) staging system for patients with intrahepatic cholangiocarcinoma: A surveillance, epidemiology, and end results (SEER) analysis. J Surg Oncol 2017;116:643-50.
- Sui K, Okabayashi T, Umeda Y, et al. Prognostic Utility of the Glasgow Prognostic Score for the Long-Term Outcomes After Liver Resection for Intrahepatic Cholangiocarcinoma: A Multi-institutional Study. World J Surg 2021;45:279-90. [Crossref] [PubMed]
- Bartsch F, Baumgart J, Hoppe-Lotichius M, et al. Intrahepatic cholangiocarcinoma - influence of resection margin and tumor distance to the liver capsule on survival. BMC Surg 2020;20:61. [Crossref] [PubMed]
- Addeo P, Jedidi I, Locicero A, et al. Prognostic Impact of Tumor Multinodularity in Intrahepatic Cholangiocarcinoma. J Gastrointest Surg 2019;23:1801-9. [Crossref] [PubMed]
- Conci S, Ruzzenente A, Viganò L, et al. Patterns of Distribution of Hepatic Nodules (Single, Satellites or Multifocal) in Intrahepatic Cholangiocarcinoma: Prognostic Impact After Surgery. Ann Surg Oncol 2018;25:3719-27. [Crossref] [PubMed]
- Reames BN, Ejaz A, Koerkamp BG, et al. Impact of major vascular resection on outcomes and survival in patients with intrahepatic cholangiocarcinoma: A multi-institutional analysis. J Surg Oncol 2017;116:133-9. [Crossref] [PubMed]
- Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol 2009;16:3048-56. [Crossref] [PubMed]
- Buettner S, Ten Cate DWG, Bagante F, et al. Survival after Resection of Multiple Tumor Foci of Intrahepatic Cholangiocarcinoma. J Gastrointest Surg 2019;23:2239-46. [Crossref] [PubMed]
- Kim Y, Spolverato G, Amini N, et al. Surgical Management of Intrahepatic Cholangiocarcinoma: Defining an Optimal Prognostic Lymph Node Stratification Schema. Ann Surg Oncol 2015;22:2772-8. [Crossref] [PubMed]
- Farges O, Fuks D, Boleslawski E, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg 2011;254:824-29; discussion 830. [Crossref] [PubMed]
- Tamandl D, Herberger B, Gruenberger B, et al. Influence of hepatic resection margin on recurrence and survival in intrahepatic cholangiocarcinoma. Ann Surg Oncol 2008;15:2787-94. [Crossref] [PubMed]
- Watanabe Y, Matsuyama Y, Izumi N, et al. Effect of surgical margin width after R0 resection for intrahepatic cholangiocarcinoma: A nationwide survey of the Liver Cancer Study Group of Japan. Surgery 2020;167:793-802. [Crossref] [PubMed]
- Kim DH, Choi DW, Choi SH, et al. Is there a role for systematic hepatic pedicle lymphadenectomy in intrahepatic cholangiocarcinoma? A review of 17 years of experience in a tertiary institution. Surgery 2015;157:666-75. [Crossref] [PubMed]
- Kizy S, Altman AM, Marmor S, et al. Surgical resection of lymph node positive intrahepatic cholangiocarcinoma may not improve survival. HPB (Oxford) 2019;21:235-41. [Crossref] [PubMed]
- Yoh T, Cauchy F, Le Roy B, et al. Prognostic value of lymphadenectomy for long-term outcomes in node-negative intrahepatic cholangiocarcinoma: A multicenter study. Surgery 2019;166:975-82. [Crossref] [PubMed]
- Bagante F, Spolverato G, Weiss M, et al. Assessment of the Lymph Node Status in Patients Undergoing Liver Resection for Intrahepatic Cholangiocarcinoma: the New Eighth Edition AJCC Staging System. J Gastrointest Surg 2018;22:52-9.
- Zhang XF, Lv Y, Weiss M, et al. Should Utilization of Lymphadenectomy Vary According to Morphologic Subtype of Intrahepatic Cholangiocarcinoma? Ann Surg Oncol 2019;26:2242-50. [Crossref] [PubMed]
- Spolverato G, Kim Y, Alexandrescu S, et al. Is Hepatic Resection for Large or Multifocal Intrahepatic Cholangiocarcinoma Justified? Results from a Multi-Institutional Collaboration. Ann Surg Oncol 2015;22:2218-25. [Crossref] [PubMed]
- Wright GP, Perkins S, Jones H, et al. Surgical Resection Does Not Improve Survival in Multifocal Intrahepatic Cholangiocarcinoma: A Comparison of Surgical Resection with Intra-Arterial Therapies. Ann Surg Oncol 2018;25:83-90. [Crossref] [PubMed]
- Kamarajah SK, Frankel TL, Sonnenday C, et al. Critical evaluation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with Hepatocellular Carcinoma (HCC): A Surveillance, Epidemiology, End Results (SEER) analysis. J Surg Oncol 2018;117:644-50.
- Ali SM, Clark CJ, Zaydfudim VM, et al. Role of major vascular resection in patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol 2013;20:2023-8. [Crossref] [PubMed]
- Nathan H, Aloia TA, Vauthey JN, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol 2009;16:14-22. [Crossref] [PubMed]
- Uenishi T, Ariizumi S, Aoki T, et al. Proposal of a new staging system for mass-forming intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2014;21:499-508. [Crossref] [PubMed]
- Farges O, Fuks D, Le Treut YP, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: By the AFC-IHCC-2009 study group. Cancer 2011;117:2170-7.
- Kang SH, Hwang S, Lee YJ, et al. Prognostic comparison of the 7th and 8th editions of the American Joint Committee on Cancer staging system for intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2018;25:240-8.
- Spolverato G, Bagante F, Weiss M, et al. Comparative performances of the 7th and the 8th editions of the American Joint Committee on Cancer staging systems for intrahepatic cholangiocarcinoma. J Surg Oncol 2017;115:696-703.
- Sasaki K, Margonis GA, Andreatos N, et al. Serum tumor markers enhance the predictive power of the AJCC and LCSGJ staging systems in resectable intrahepatic cholangiocarcinoma. HPB (Oxford) 2018;20:956-65. [Crossref] [PubMed]
- Yeh CN, Wang SY, Chen YY, et al. A Prognostic Nomogram for Overall Survival of Patients After Hepatectomy for Intrahepatic Cholangiocarcinoma. Anticancer Res 2016;36:4249-58. [PubMed]
- Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. [Crossref] [PubMed]
- Hyder O, Marques H, Pulitano C, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg 2014;149:432-8. [Crossref] [PubMed]
- Yoh T, Hatano E, Seo S, et al. Long-Term Survival of Recurrent Intrahepatic Cholangiocarcinoma: The Impact and Selection of Repeat Surgery. World J Surg 2018;42:1848-56. [Crossref] [PubMed]
- Spolverato G, Kim Y, Alexandrescu S, et al. Management and Outcomes of Patients with Recurrent Intrahepatic Cholangiocarcinoma Following Previous Curative-Intent Surgical Resection. Ann Surg Oncol 2016;23:235-43. [Crossref] [PubMed]
- Si A, Li J, Xing X, et al. Effectiveness of repeat hepatic resection for patients with recurrent intrahepatic cholangiocarcinoma: Factors associated with long-term outcomes. Surgery 2017;161:897-908. [Crossref] [PubMed]
- Bartsch F, Paschold M, Baumgart J, et al. Surgical Resection for Recurrent Intrahepatic Cholangiocarcinoma. World J Surg 2019;43:1105-16. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Levi Sandri GB, Spoletini G, Mascianà G, et al. The role of minimally invasive surgery in the treatment of cholangiocarcinoma. Eur J Surg Oncol 2017;43:1617-21. [Crossref] [PubMed]
- Uy BJ, Han HS, Yoon YS, et al. Laparoscopic liver resection for intrahepatic cholangiocarcinoma. J Laparoendosc Adv Surg Tech A 2015;25:272-7. [Crossref] [PubMed]
- Lee W, Park JH, Kim JY, et al. Comparison of perioperative and oncologic outcomes between open and laparoscopic liver resection for intrahepatic cholangiocarcinoma. Surg Endosc 2016;30:4835-40. [Crossref] [PubMed]
- Wei F, Lu C, Cai L, et al. Can laparoscopic liver resection provide a favorable option for patients with large or multiple intrahepatic cholangiocarcinomas? Surg Endosc 2017;31:3646-55. [Crossref] [PubMed]
- Ratti F, Cipriani F, Ariotti R, et al. Safety and feasibility of laparoscopic liver resection with associated lymphadenectomy for intrahepatic cholangiocarcinoma: a propensity score-based case-matched analysis from a single institution. Surg Endosc 2016;30:1999-2010. [Crossref] [PubMed]
- Guerrini GP, Esposito G, Tarantino G, et al. Laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma: the first meta-analysis. Langenbecks Arch Surg 2020;405:265-75. [Crossref] [PubMed]
- Martin SP, Drake J, Wach MM, et al. Laparoscopic Approach to Intrahepatic Cholangiocarcinoma is Associated with an Exacerbation of Inadequate Nodal Staging. Ann Surg Oncol 2019;26:1851-7. [Crossref] [PubMed]
- Xu Y, Wang H, Ji W, et al. Robotic radical resection for hilar cholangiocarcinoma: perioperative and long-term outcomes of an initial series. Surg Endosc 2016;30:3060-70. [Crossref] [PubMed]
- Liu R, Wakabayashi G, Kim HJ, et al. International consensus statement on robotic hepatectomy surgery in 2018. World J Gastroenterol 2019;25:1432-44. [Crossref] [PubMed]
- Khan S, Beard RE, Kingham PT, et al. Long-Term Oncologic Outcomes Following Robotic Liver Resections for Primary Hepatobiliary Malignancies: A Multicenter Study. Ann Surg Oncol 2018;25:2652-60. [Crossref] [PubMed]
- Tsilimigras DI, Moris D, Vagios S, et al. Safety and oncologic outcomes of robotic liver resections: A systematic review. J Surg Oncol 2018;117:1517-30. [Crossref] [PubMed]
- Tian Y, Liu L, Yeolkar NV, et al. Diagnostic role of staging laparoscopy in a subset of biliary cancers: a meta-analysis. ANZ J Surg 2017;87:22-7. [Crossref] [PubMed]
- Davidson JT, Jin LX, Krasnick B, et al. Staging laparoscopy among three subtypes of extra-hepatic biliary malignancy: a 15-year experience from 10 institutions. J Surg Oncol 2019;119:288-94. [Crossref] [PubMed]
- D’Angelica M, Fong Y, Weber S, et al. The role of staging laparoscopy in hepatobiliary malignancy: prospective analysis of 401 cases. Ann Surg Oncol 2003;10:183-9. [Crossref] [PubMed]
- Sur MD, In H, Sharpe SM, et al. Defining the Benefit of Adjuvant Therapy Following Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2015;22:2209-17. [Crossref] [PubMed]
- Reames BN, Bagante F, Ejaz A, et al. Impact of adjuvant chemotherapy on survival in patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis. HPB (Oxford) 2017;19:901-9. [Crossref] [PubMed]
- Schweitzer N, Weber T, Kirstein MM, et al. The effect of adjuvant chemotherapy in patients with intrahepatic cholangiocarcinoma: a matched pair analysis. J Cancer Res Clin Oncol 2017;143:1347-55. [Crossref] [PubMed]
- Ma KW, Cheung TT, Leung B, et al. Adjuvant chemotherapy improves oncological outcomes of resectable intrahepatic cholangiocarcinoma: A meta-analysis. Medicine (Baltimore) 2019;98:e14013 [Crossref] [PubMed]
- Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol 2019;37:658-67. [Crossref] [PubMed]
- Ebata T, Hirano S, Konishi M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg 2018;105:192-202. [Crossref] [PubMed]
- Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019;20:663-73. [Crossref] [PubMed]
- Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. Jama 2012;308:147-56. [Crossref] [PubMed]
- Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J Clin Oncol 2019;37:1015-27. [Crossref] [PubMed]
- Stein A, Arnold D, Bridgewater J, et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer 2015;15:564. [Crossref] [PubMed]
- Buettner S, Koerkamp BG, Ejaz A, et al. The effect of preoperative chemotherapy treatment in surgically treated intrahepatic cholangiocarcinoma patients-A multi-institutional analysis. J Surg Oncol 2017;115:312-8. [Crossref] [PubMed]
- Le Roy B, Gelli M, Pittau G, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg 2018;105:839-47. [Crossref] [PubMed]
- Boudjema K, Edeline J. ASO Author Reflections: Intrahepatic Cholangiocarcinoma: Downstaging Strategies Open the Gate to Surgery and Cure. Ann Surg Oncol 2020;27:3738-9. [Crossref] [PubMed]
- Riby D, Mazzotta AD, Bergeat D, et al. Downstaging with Radioembolization or Chemotherapy for Initially Unresectable Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2020;27:3729-37. [Crossref] [PubMed]
- Rayar M, Sulpice L, Edeline J, et al. Intra-arterial yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol 2015;22:3102-8. [Crossref] [PubMed]
- Edeline J, Touchefeu Y, Guiu B, et al. Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol 2019;6:51-9. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [Crossref] [PubMed]
- Pichlmayr R, Weimann A, Oldhafer KJ, et al. Role of liver transplantation in the treatment of unresectable liver cancer. World J Surg 1995;19:807-13. [Crossref] [PubMed]
- Goldstein RM, Stone M, Tillery GW, et al. Is liver transplantation indicated for cholangiocarcinoma? Am J Surg 1993;166:768-71; discussion 771-2. [Crossref] [PubMed]
- Casavilla FA, Marsh JW, Iwatsuki S, et al. Hepatic resection and transplantation for peripheral cholangiocarcinoma. J Am Coll Surg 1997;185:429-36. [Crossref] [PubMed]
- Ghali P, Marotta PJ, Yoshida EM, et al. Liver transplantation for incidental cholangiocarcinoma: analysis of the Canadian experience. Liver Transpl 2005;11:1412-6. [Crossref] [PubMed]
- Brandsaeter B, Isoniemi H, Broome U, et al. Liver transplantation for primary sclerosing cholangitis; predictors and consequences of hepatobiliary malignancy. J Hepatol 2004;40:815-22. [Crossref] [PubMed]
- Becker NS, Rodriguez JA, Barshes NR, et al. Outcomes analysis for 280 patients with cholangiocarcinoma treated with liver transplantation over an 18-year period. J Gastrointest Surg 2008;12:117-22. [Crossref] [PubMed]
- Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation 2000;69:1633-7. [Crossref] [PubMed]
- Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168-79. [Crossref] [PubMed]
- Sapisochin G, Rodriguez de Lope C, Gastaca M, et al. “Very early” intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am J Transplant 2014;14:660-7. [Crossref] [PubMed]
- Sapisochin G, Facciuto M, Rubbia-Brandt L, et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology 2016;64:1178-88. [Crossref] [PubMed]
- Sapisochin G, de Lope CR, Gastaca M, et al. Intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma in patients undergoing liver transplantation: a Spanish matched cohort multicenter study. Ann Surg 2014;259:944-52. [Crossref] [PubMed]
- Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012;143:88-98.e3; quiz e14. [Crossref] [PubMed]
- Lunsford KE, Javle M, Heyne K, et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol 2018;3:337-48. [Crossref] [PubMed]
- Hong JC, Petrowsky H, Kaldas FM, et al. Predictive index for tumor recurrence after liver transplantation for locally advanced intrahepatic and hilar cholangiocarcinoma. J Am Coll Surg 2011;212:514-20; discussion 520-1. [Crossref] [PubMed]
- Hong JC, Jones CM, Duffy JP, et al. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: a 24-year experience in a single center. Arch Surg 2011;146:683-9. [Crossref] [PubMed]
- Mazzaferro V, Gorgen A, Roayaie S, et al. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol 2020;72:364-77. [Crossref] [PubMed]
- Zhu AX, Borger DR, Kim Y, et al. Genomic profiling of intrahepatic cholangiocarcinoma: refining prognosis and identifying therapeutic targets. Ann Surg Oncol 2014;21:3827-34. [Crossref] [PubMed]
Cite this article as: Lei HJ, Shyr YM, Hsia CY, Chen MH, Huang YH, Chau GY. Surgical management of intrahepatic cholangiocarcinoma: a narrative review. Dig Med Res 2021;4:4.


