
A woman with anti-mitochondrial antibody-negative primary biliary cholangitis for a long-term intermittent elevated hepatic enzymes: a case report
Introduction
Primary biliary cholangitis (PBC), formerly named primary biliary cirrhosis, is a chronic progressive cholestatic liver disease, which is histologically characterized by an autoimmune and nonsuppurative inflammatory destruction of small bile ducts resulting in liver fibrosis and cirrhosis (1-4). It was first described in 1761 and the histology of non-obstructive biliary cirrhosis was no reported until 1851 (1). Seven years later, the PBC related autoantigens were first discovered since the sera of patients with PBC had high titers of autoantibodies to the organ and tissue antigens (4). In 1965, Walker described the characteristic fluorescence pattern due to reactivity to antigens present in cytoplasmic organelles, especially mitochondria. This was the first time the association of anti-mitochondrial antibody (AMA) with PBC was recognized (5).
To date, nine AMA-target antigens, which are divided into M1–M9, have been detected, with only the M2 confirmed to be related to the PBC (6). It is located in the inner mitochondrial membrane and identified as one of the 2-oxo-acid dehydrogenase complexes (2-OADC), a component of mitochondrial energy metabolism (7). Furthermore, since it was detected in 90–95% of PBC patients, with elevated serum alkaline phosphatase (ALP) and typical histopathology, AMA acts as an important marker to the criteria for diagnosing PBC (8-10). The sensitivity of AMA is as high as 95% and the specificity even reaches 100% (11). The presence of AMA sometimes indicates PBC before its onset of clinical manifestation and other biochemical or histopathologic evidence, which is proven by some prospective studies (1,11,12).
Strangely, a limited number of patients have undetectable AMA while presenting clinical or histological manifestations of PBC. Here, after the written consent has been obtained from the subject, we presented the following rare and complicated case of AMA-negative PBC.
We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/dmr-20-129).
Case presentation
A 48-year-old woman was admitted to our hospital because of the intermittent elevation of hepatic enzymes for as long as 8 years. She rarely drunk and never took any medicine. Alanine aminotransferase (ALT) was firstly found at an abnormally high level of 80 IU/L and normalized after the treatment with polyene phosphatidylcholine for 2 weeks. Abdominal ultrasonic and computed tomography imaging, as well as other serological tests like immunoglobulins of cytomegalovirus and Epstein-Barr virus, hepatitis B virus antigen, hepatitis C virus antibody, ceruloplasmin, AMA were performed, but no clear etiology was found at that time. She had no discomfort such as pruritus, fatigue, jaundice, anorexia or other gastrointestinal symptoms. In the last 3 years, she felt slightly tired briefly every time her serum ALT was elevated, which reached a peak of 280 IU/L. Whenever she took polyene phosphatidylcholine, her ALT reverted to normal state.
On physical examination, her BMI was 23.2 kg/m2 and abdominal circumference was 79 cm. Neither stigmata of chronic liver disease nor Kayser-Fleischer rings were found. However, xerophthalmia was diagnosed inexplicably with the ophthalmological testing. A soft 2 cm lymph node in the posterior lateral superficial cervical nodes was also palpable.
The liver function profile showed normal total bilirubin, the ALT was 70 U/L (normal range, 7 to 40), AST was 51 U/L (normal range, 13 to 35), ALP was 128 U/L (normal range, 35 to 100) and γ-glutamyl-transpeptidase (GGT) was 184 U/L (normal range, 7 to 45). Serum total IgG and IgM levels were also higher than normal, 25.88 g/L (normal range, 7 to 16) and 5.64 g/L (normal range, 0.4 to 2.8). However, IgG4 was normal, 0.92 g/L. Serum IgA, IgE, and complement 3 and 4 were normal. As what she had taken before, the serum AMA-M2 was negative when tested through an immunoblotting (IB). While the titers of antinuclear antibody (ANA) was 1:320 in a way of indirect immunofluorescence (IIF) (normal range, negative), rheumatoid factor (RF) was 74 IU/mL (normal range, <30), thyroglobulin antibody was 140.61 IU/mL (normal range, <4.11) and erythrocyte sedimentation rate (ESR) was 101 mm/h (normal range, 0 to 20).
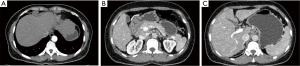
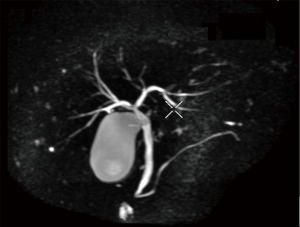
Computed tomography and magnetic resonance imaging showed slightly rough edge of the liver, indicating early stage cirrhosis. Significantly, lymphoglandula hyperplasia were seen in multiple sites including the portal area of liver, perihepatic area, omental bursa and superior phrenic lymph nodes which ranged from 7 to 23 mm in diameter (Figure 1).The intrahepatic bile ducts and common hepatic duct were unremarkable in the imaging of magnetic resonance cholangiopancreatography (MRCP) (Figure 2).
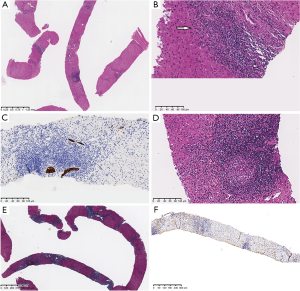
The histopathologic examination of a cervical lymph node indicated an inflammatory reactive hyperplasia. Subsequently, we performed a percutaneous liver biopsy through an ultrasound-guided fine needle aspiration. Mild proliferation of smaller bile ductulus and bridging fibrosis in portal areas were found by histology. Besides, inflammatory cells infiltrated both the portal areas and bill duct wall, in some of which even granulomas formation was shown. Also, the IgG4 existence was excluded by the immunohistochemical staining (Figure 3).
In short conclusion, AMA-negative PBC in a female patient with long-term elevated ALT was diagnosed. She was regularly treated by ursodesoxycholic acid 250 mg twice per day as well as polyene phosphatidylcholine 456 mg three times a day. We kept the follow-up which showed that pharmacologic therapy produced no side effects and ensured the compliance. Her liver function profile surveillance also appeared increasingly improved. The latest test after half-year therapy showed decreased ALT, AST, ALP and GGT (ALT: 57 U/L, AST: 43 U/L, ALP: 103 U/L, GGT: 71 U/L).
All procedures performed in this case were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013).
Discussion
A middle-aged woman with intermittent elevated hepatic enzymes for a long time was reported. Though suffering for a long period, she never took other medicine except docetaxel phosphatidylcholine and live a healthy lifestyle. As a result, it’s feasible for us to exclude some common reasons of hepatic damage like drugs, alcohol or metabolism. As mentioned above, the diagnosis of PBC can be established when two of the three criteria are met, which including presence of AMA, ALP elevation and characteristic histologic appearance (10). The patient was prone to misdiagnosis due to the deficiency of AMA-M2 and apparent clinical manifestations such as pruritus or jaundice. Moreover, the other kinds of autoantibodies were found abnormally elevated, which caused the case even more complexed and difficult to make a diagnose. The reasons of intermittent hepatic injury were hard to distinguish between primary hepatic diseases and complications of systemic immunological/non-immunological diseases. In order to learn more about the hepatic injury, the percutaneous liver biopsy was performed, discovering PBC characteristic histological appearance. On the base of histological evidence and the elevated ALP, the patient was diagnosed with PBC finally. Then she was treated with ursodeoxycholic acid formally, the patient said she felt nothing uncomfortable even no more tired after she got the treatment. She was satisfactory about the outcome of the treatment and willing to sustain the regular pharmaceutical therapy.
The presence of AMA negative PBC has something to do with the limitation of detection techniques. To date, with the development of immunological tests and histopathological imaging targeting the detailed structure of AMA-specific antigens more precisely, the AMA-negative PBC population has been progressively reduced (2,7). AMA is detected mainly by IIF assay, currently the most widely accepted techniques and the gold standard for the detection of AMA by well-trained technicians (4,13). It detects full AMA as a pattern and provides additional information such as the other PBC-related ANAs (12). After the 2-OADC is proven to be composed of three enzymes: pyruvate dehydrogenase complex-E2 (PDC-E2), branched chain2-oxo-acid dehydrogenase (BCOADC-E2), and 2-oxo-glutarate dehydrogenase (OGDC-E2), the other two AMA detection assays, enzyme-linked immunosorbent assay (ELISA) and IB have usually been used (13). Many studies showed that IIF is easily misdiagnosed by the technicians’ skills, substrates of tissue extract as well as confusion with other cytoplasmatic antibodies, known as false negative (12,13). The additional use of ELISA and IB increases the sensitivity and specificity for AMA detection (2,14,15). In contrast to IIF, they mainly detect the specific one or combine pre-selected subtypes of M2 antigens (4,13). Nowadays, there are many kinds of ELISA and IB kits classified by different antigens and substrates. Some patients proven to be negative by IIF will be AMA seropositive thorough the other two assays, ranging from 24–100% in different studies (7,13,16-18). In this case, negative result of the patient’s AMA was credible because it was detected through the IB which had higher sensitivity and specificity as analysis before.
Apart from AMA, several types of PBC related to ANAs have been confirmed and become an important supplemental hallmark for PBC (13). They were first detected in the 1950s and then a number of nuclear structures were recognized as specific targets (9). Like AMA, the detection of ANA is mainly tested through IIF, ELISA and IB. One-third of PBC was ANA detectable solely by IIF and furtherly, it would be 50% to 70% through all three assays (19,20). Two IIF patterns have been identified as the “multiple nuclear dots” (MND) and “nuclear membrane (rim-like)”. More specifically detected by IB and ELISA, the MND pattern to nuclear antigens are Sp100, Sp140, promyelocytic leukemia nuclear body proteins (PML) and small ubiquitin-like modifiers (SUMO); lamin-B-receptor as well as two components of the nuclear pore complexes: gp210 and p62, are associated with the rim-like pattern (4,8,12,13,19). The diagnostic sensitivity of ANA is only 30%, while it has the specificity reaching 99% (13). It appears more common in the AMA-negative PBC patients, being a surrogate marker with excellent diagnostic values for such rare subtype (4,6-8,12,19,21). Interestingly, the patient we reported here was diagnosed with PBC, whose AMA was negative while ANA was detectable.
Between the two groups with AMA positive and negative PBC, the difference of clinical manifestations and biochemical features is still controversial despite abundant studies. Oertelt et al. found no significant difference in age, disease duration, biochemical features and histological features in two PBC groups between 90 cases of AMA positive and 30 patients of AMA negative (7). The outcomes of the other study by Liu et al. were similar, including the response to a 1-year ursodeoxycholic acid treatment (22). While a retrospective chart review of 142 PBC patients at the Mayo Clinic showed a significantly reduced survival free of complications for the AMA negative after the follow-up of at least 7.5 years, there was no difference in terms of age, biochemical index or length of follow-up (23). Similar, an American national multicenter clinical trial with 535 PBC patients discovered nothing special after they compared the severity of disease activity, pruritus, ascites and varices. Hepatic encephalopathy as well as icterus is more frequent in AMA-negative, attributed to the difficulty and delay in the diagnosis for this group is attributed (24). One Japanese study of 2,419 patients with PBC, including 470 AMA negative patients, showed that the incidence rate of pruritus was less frequent, the levels of ALP, γ-GTP, and IgM were lower in the AMA negative group, but the presence of some complicated autoimmune diseases like Sjögren’s syndrome, rheumatoid arthritis, scleroderma is higher compared to the AMA positive group (25). Furthermore, Deng et al. first used serological comparative proteomics containing 14 proteins to explore the differences. The level of vitronectin was higher in the AMA-negative, which related to more severe bile duct destruction (26). The histological feature has been also found by the other study (27).
Above all, difference of clinical manifestations and biochemical features between two groups is still controversial. While the patient we reported here did suffer xerophthalmia, a series of reactive lymph node hyperplasia and high-titer autoantibodies like ANA, RF, thyroglobulin antibody. These symptoms indicated some other autoimmune diseases such as Sjögren’s syndrome and rheumatoid arthritis, similarly to the previous Japanese study. To learn whether autoimmune diseases happen, the follow-up for this patient was necessary.
The patient received detailed examinations when she was admitted in our hospital. As a result, more comprehensive information was got comparing to other similar previous studies. It helped us to learn more about this rare subtype of PBC. Besides, it should be noticed the case had the limitation. She had been suffering the disease for a long-time period and it’s impossible for her to recall every kind of diet or life style during these days. Although we considered there was little factors causing hepatic damage except the original disease as mentioned before, it’s difficult to confirm completely.
Here, we reported a case of AMA-negative PBC and further elucidated factors relating to onset of this rare disease. Other similar studies in its characters were reviewed and compared to this case. We learned a lot from all mentioned above. Firstly, it reminded us the AMA was not essential to PBC and whenever exclude other patients from it, the other test like histologic examination was crucial. Secondly, when AMA-negative PBC was detected, the patients’ clinical manifestations and biochemical features might also appear differently and the concomitant systemic autoimmune diseases were more common.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/dmr-20-129
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-20-129). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this case were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lleo A, Marzorati S, Anaya JM, et al. Primary biliary cholangitis: a comprehensive overview. Hepatol Int 2017;11:485-99. [Crossref] [PubMed]
- Bizzaro N, Covini G, Rosina F, et al. Overcoming a "probable" diagnosis in antimitochondrial antibody negative primary biliary cirrhosis: study of 100 sera and review of the literature. Clin Rev Allergy Immunol 2012;42:288-97. [Crossref] [PubMed]
- Muratori P, Muratori L, Ferrari R, et al. Characterization and clinical impact of antinuclear antibodies in primary biliary cirrhosis. Am J Gastroenterol 2003;98:431-7. [Crossref] [PubMed]
- Bogdanos DP, Komorowski L. Disease-specific autoantibodies in primary biliary cirrhosis. Clin Chim Acta 2011;412:502-12. [Crossref] [PubMed]
- Walker JG, Doniach D, Roitt IM, et al. Serological tests in diagnosis of primary biliary cirrhosis. Lancet 1965;1:827-31. [Crossref] [PubMed]
- Alfano AM, Romito A, Marchese C, et al. Diagnostic accuracy of two tests for determination of anti-m2 in the diagnosis of primary biliary cirrhosis: is it possible to predict the course of the disease? Immunol Res 2017;65:299-306. [Crossref] [PubMed]
- Oertelt S, Rieger R, Selmi C, et al. A sensitive bead assay for antimitochondrial antibodies: chipping away at AMA-negative primary biliary cirrhosis. Hepatology 2007;45:659-65. [Crossref] [PubMed]
- de Liso F, Matinato C, Ronchi M, et al. The diagnostic accuracy of biomarkers for diagnosis of primary biliary cholangitis (PBC) in anti-mitochondrial antibody (AMA)-negative PBC patients: a review of literature. Clin Chem Lab Med 2017;56:25-31. [Crossref] [PubMed]
- Invernizzi P, Selmi C, Ranftler C, et al. Antinuclear antibodies in primary biliary cirrhosis. Semin Liver Dis 2005;25:298-310. [Crossref] [PubMed]
- Lindor KD, Bowlus CL, Boyer J, et al. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2019;69:394-419. [PubMed]
- Uibo R, Kisand K, Yang CY, et al. Primary biliary cirrhosis: a multi-faced interactive disease involving genetics, environment and the immune response. APMIS 2012;120:857-71. [Crossref] [PubMed]
- Sebode M, Weiler-Normann C, Liwinski T, et al. Autoantibodies in autoimmune liver disease-clinical and diagnostic relevance. Front Immunol 2018;9:609. [Crossref] [PubMed]
- Ozaslan E, Efe C, Gokbulut Ozaslan N. The diagnosis of antimitochondrial antibody-negative primary biliary cholangitis. Clin Res Hepatol Gastroenterol 2016;40:553-61. [Crossref] [PubMed]
- Gershwin ME, Coppel RL, Mackay IR. Primary biliary cirrhosis and mitochondrial autoantigens--insights from molecular biology. Hepatology 1988;8:147-51. [Crossref] [PubMed]
- Heseltine L, Turner IB, Fussey SP, et al. Primary biliary cirrhosis. Quantitation of autoantibodies to purified mitochondrial enzymes and correlation with disease progression. Gastroenterology 1990;99:1786-92. [Crossref] [PubMed]
- Miyakawa H, Tanaka A, Kikuchi K, et al. Detection of antimitochondrial autoantibodies in immunofluorescent AMA-negative patients with primary biliary cirrhosis using recombinant autoantigens. Hepatology 2001;34:243-8. [Crossref] [PubMed]
- Moteki S, Leung PS, Coppel RL, et al. Use of a designer triple expression hybrid clone for three different lipoyl domain for the detection of antimitochondrial autoantibodies. Hepatology 1996;24:97-103. [Crossref] [PubMed]
- Oertelt S, Ridgway WM, Ansari AA, et al. Murine models of primary biliary cirrhosis: comparisons and contrasts. Hepatol Res 2007;37:S365-9. [Crossref] [PubMed]
- Granito A, Muratori P, Quarneti C, et al. Antinuclear antibodies as ancillary markers in primary biliary cirrhosis. Expert Rev Mol Diagn 2012;12:65-74. [Crossref] [PubMed]
- Invernizzi P, Podda M, Battezzati PM, et al. Autoantibodies against nuclear pore complexes are associated with more active and severe liver disease in primary biliary cirrhosis. J Hepatol 2001;34:366-72. [Crossref] [PubMed]
- Hu S, Zhao F, Wang Q, et al. The accuracy of the anti-mitochondrial antibody and the M2 subtype test for diagnosis of primary biliary cirrhosis: a meta-analysis. Clin Chem Lab Med 2014;52:1533-42. [Crossref] [PubMed]
- Liu B, Shi XH, Zhang FC, et al. Antimitochondrial antibody-negative primary biliary cirrhosis: a subset of primary biliary cirrhosis. Liver Int 2008;28:233-9. [Crossref] [PubMed]
- Juliusson G, Imam M, Björnsson ES, et al. Long-term outcomes in antimitochondrial antibody negative primary biliary cirrhosis. Scand J Gastroenterol 2016;51:745-52. [Crossref] [PubMed]
- Peters MG, Di Bisceglie AM, Kowdley KV, et al. Differences between Caucasian, African American, and Hispanic patients with primary biliary cirrhosis in the United States. Hepatology 2007;46:769-75. [Crossref] [PubMed]
- Sakauchi F, Mori M, Zeniya M, et al. Antimitochondrial antibody negative primary biliary cirrhosis in Japan: utilization of clinical data when patients applied to receive public financial aid. J Epidemiol 2006;16:30-4. [Crossref] [PubMed]
- Deng C, Hu C, Wang L, et al. Serological comparative proteomics analysis of mitochondrial autoantibody-negative and -positive primary biliary cirrhosis. Electrophoresis 2015;36:1588-95. [Crossref] [PubMed]
- Jin Q, Moritoki Y, Lleo A, et al. Comparative analysis of portal cell infiltrates in antimitochondrial autoantibody-positive versus antimitochondrial autoantibody-negative primary biliary cirrhosis. Hepatology 2012;55:1495-506. [Crossref] [PubMed]
Cite this article as: Zhan SK, Zhu JF, Deng X, Chueng EC, Chen YP. A woman with anti-mitochondrial antibody-negative primary biliary cholangitis for a long-term intermittent elevated hepatic enzymes: a case report. Dig Med Res 2021;4:20.


