
Role of imaging and synoptic MRI reporting in determining optimal management paradigm for rectal cancer: a narrative review
Introduction
Rectal cancer is one of the major causes of cancer-related mortality worldwide. Magnetic resonance imaging (MRI) plays an essential role in the local staging of rectal cancer and should routinely be performed for primary staging as well as for post-treatment assessment (1). The primary goal of MRI staging of rectal tumours prior to treatment is to identify prognostic factors which enable the multidisciplinary team (MDT) to tailor treatments based on individual risks (2).
The initial MRI staging should be documented with a prefix “mr”, not to be conflated with the pathological staging denoted with a prefix “p” (3). MRI of the rectum identifies patients who will benefit from neoadjuvant therapy prior to surgery to minimise postoperative recurrence and assists in planning the optimal surgical approach (4). Standardised synoptic MRI reports, incorporating evidence-based key prognostic information, ensure that all of the relevant information is included to allow correct treatment selection and facilitate discussion with patients to better understand how these factors impact their prognosis and management.
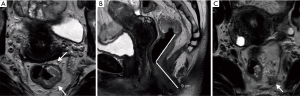
In primary staging, rectal MRI provides information about the tumour location and morphology. The radiologist is able to accurately assess MRI T staging (mrT) (3), ascertain whether there is extramural vascular invasion (mrEMVI) and describe the relationship of the tumour with surrounding structures, such as the sphincter complex and the potential surgical circumferential resection margin (CRM) (5) (Figure 1). These features help diagnose locally advanced rectal tumours for which neoadjuvant chemoradiotherapy is indicated (6). The adoption of total mesorectal excision (TME) as the standard treatment of rectal cancer and the use of neoadjuvant chemoradiotherapy (CRT) for patients with locally advanced rectal cancer has led to significant gains in local disease control (6).
In this review, the role and accuracy of MRI in the local staging of rectal cancer both at baseline and after neoadjuvant treatment, the ideal MR imaging protocol and the benefits of proforma reporting will be discussed. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/dmr-20-147).
Protocol
Good quality MRI images maximise the benefits achieved with rectal MRI allowing the radiologist to accurately characterise anatomic structures and their relationship with the tumour (6). High-resolution T2-weighted sequences are crucial for evaluating rectal tumours (2).
The standard rectal MRI protocol for evaluating rectal cancer includes acquiring high spatial resolution two-dimensional (2D) fast spin echo (FSE) T2-weighted sequences without fat suppression, with a small field of view and a slice thickness of 3 mm in the oblique axial plane, sagittal plane and oblique coronal plane.
FSE T2-weighted MRI without fat suppression and with a large field of view performed in the axial plane of the entire pelvis, from the aortic bifurcation to the sphincter, permits evaluation of distant lymph nodes. In the sagittal plane, FSE T2-weighted MRI localises the primary tumour, which enables the measurement of its craniocaudal length and its height above the anal verge (6). Intravenous contrast is not required as enhanced T1-weighted imaging does not improve the diagnostic accuracy of local staging of rectal cancer (7).
Currently, functional and molecular MR imaging techniques are not, as yet, routinely used in the detection of rectal cancer. They may play a role in the near future for the assessment of tumour characteristics, such as tumour heterogeneity, and may provide prognostic information to guide treatment decisions. The literature regarding the role of other techniques such as MR Spectroscopy and blood oxygenation level-dependent-MRI is sparse (8)
Although computed tomography (CT) has the advantages of fast scan times and being widely accessible, a meta-analysis by Bipat et al. exploring the accuracy of different imaging modalities for local staging of rectal cancer found that CT was of limited use. The different layers of the rectal wall are less well differentiated on CT (9). In addition, CT cannot distinguish between tumour and peritumoral desmoplastic reaction, which could potentially lead to over-staging (10).
The role of synoptic reporting in determining optimal management paradigm for rectal cancer
The systematic assessment and reporting of the initial staging MRI guides MDT discussion and helps stratify patients for neoadjuvant chemoradiotherapy or surgery, avoiding over-treatment and reducing CRM positive resections (6,7,11-15).
Synoptic radiology reporting for MRI rectal cancer provides a complete and accurate assessment of the relevant prognostic factors (1,11,16). There is evidence that MRI reports using free text do not always capture the essential data required to tailor treatment options based on imaging findings (16-19).
In an audit comparing the reporting of initial staging MRI scans for rectal cancer, Siddiqui et al. reported that the proportion of essential prognostic items reported in free text reports was 69% compared to 97% when a synoptic report was utilised (17). In the setting of evaluation of locally advanced tumour for beyond TME disease, the proportion of reports containing the required data was 10% in free text reports compared to 30% when proformas were used. The audit found that the participating radiologists were more likely to use the provided synoptic report when it was incorporated into the official guidelines.
In a prospective multicentre non-blinded interventional study across 21 centres in the UK, Patel et al. studied the completeness of radiological cancer staging reports using synoptic reporting (16). They found that free text reports contained 48.7% of the essential staging items compared to 87.3% in the synoptic report. This finding was consistent across all cancer types.
Synoptic reports are accurate (7) and contain more of the relevant items that are considered important to clinicians for planning management and outcomes (16-19), both for rectal cancer and other malignancies (16). Every item in the synoptic report provides valuable information to different specialists in the multidisciplinary team. For instance, the height of the tumour above the anal verge is of particular importance to the radiotherapist for radiotherapy planning. Likewise, knowledge of mrCRM involvement is crucial to the colorectal surgeon and will impact on operative management. Report accuracy is further increased with radiologist participation in MDTs, consensus reading, webinars and workshops (16-18,20).
Another advantage of the synoptic reports is that it provides a comprehensive checklist for trainees, where they can identify their own strengths and weaknesses in reporting each of the key items.
Mandating the use of synoptic reporting can be challenging. Synoptic reporting may be more time consuming than free text reports as the former requires documentation of negative findings, which would simply be omitted in free text reports. There is limited mechanism for documenting equivocal findings, such as when prominent desmoplastic reaction may mimic extramural tumour extension or when the tumour margins are difficult to define due to motion artefact. Furthermore, there may be technical difficulties integrating proforma report templates into existing radiology information systems (RIS) depending on the institution (16).
Several recommendations and guides for structured reports have been proposed following trials and audits (1,7,12,15). We recommend the proforma used by the Royal Marsden Hospital (Appendix 1).
Key items in the synoptic report and the accuracy of MRI for these prognostic indicators
The synoptic report should contain a minimum set of data. The following are key prognostic items that should be included in all reports as seen in the proforma in Appendix 1.
Morphology of the tumour
The morphology of the tumour should be described, such as annular/semi-annular, polypoidal and the presence of ulceration which usually assists in localising the advancing edge of the tumour (Figure 1A). The position of the advancing edge can be described using a clock face. Low anterior tumours pose a greater risk of positive CRM at surgery (21). It should be noted whether the tumour demonstrates high T2 signal suggestive of mucinous histology or a large submucosal component suspicious for a signet cell pathology.
Tumour height
The height of the tumour measured from the anal verge and relationship with the peritoneal reflection is required (Figure 1B). Tumours can be classified according to the location of the tumour in craniocaudal direction from the anal verge. Upper rectal tumours are located 10–15 cm from the anal verge, mid rectum 5–10 cm from anal verge and lower rectum less than 5 cm from anal verge. This finding is used to determine the best surgical approach.
The anterior wall of the upper rectum is covered by the peritoneal reflection; the point of attachment occurs at a variable height. Assessment of involvement of the peritoneal reflection is important due to the increased risk of trans coelomic spread (22). The middle third is typically entirely encircled by mesorectal fat and may undergo TME with sphincter preserving surgery (23).
Low rectal tumours are managed differently. The mrT category is more applicable to mid- and high rectal cancers, whereas for low rectal tumours located within 5 cm of the anal verge, an anatomical description of local tumour extent is more relevant than stage alone due to the close proximity to the anal sphincter complex (6). In addition, tumours in the lower rectum can easily invade surrounding structures due to the tapering of the mesorectum toward the rostral margin of the anal canal (2).
MRI plays an important role in determining the relationship of the tumour to the internal sphincter muscle, intersphincteric plane, external sphincter and the pelvic floor (levator ani muscle) (Figure 2). For low rectal tumours, Stage 1 refers to tumour confined to bowel wall but does not extend through the full thickness of the muscle. Stage 2 describes replacement of the muscle without extension into the intersphincteric plane, with at least 1 mm distance to the levator. The mrCRM is preserved and the patient may be offered TME surgery, avoiding extra-levator abdominal perineal excision (ELAPE). In Stage 3, the tumour invades the intersphincteric plane or lies within 1 mm of levator muscle. In Stage 4, the tumour invades the external anal sphincter and is within 1 mm of the levator or beyond the levator muscle (2). For both stage 3 and 4 disease, patients require ELAPE to achieve adequate oncological resection.
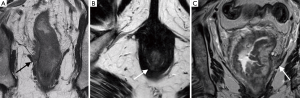
This information influences surgical approach, which aims to achieve clear radial and distal margins (24) as well as to optimise functional outcome, with regards to sphincter preservation (15). In a selected group of patients, chemoradiotherapy with delayed surgery increases the likelihood of preserving sphincter function due to a reduction in tumour size and a downstaging effect of the tumour, with consequent improved resectability (2).
The craniocaudal extent and the maximum tumour thickness is documented to determine the burden of disease and helps guide the choice of treatment.
mrT Staging
The mrT stage of a rectal cancer is assessed by the depth of tumour extension into the rectal wall, the distance of spread beyond the wall into the mesorectum and the presence of invasion into adjacent structures. Accurate assessment of mrT stage of a rectal tumour guides treatment and provides prognostic information (25).
- mrT1 tumours are those that invade the submucosa without extension into the muscularis propria (Figure 3).
- mrT2 tumours extend into the muscularis propria without extension to the mesorectal fat (Figure 4).
- mrT3 tumours extend beyond the muscularis propria into the mesorectal fat, with substages depending on the distance of extension into the mesorectal fat, measured from the outer edge of the muscularis propria:
- mrT3a less than 1 mm spread
- mrT3b 1–5 mm
- mrT3c 5–15 mm
- mrT3d greater than 15 mm (Figure 5).
- mrT4 tumours are distinguished according to invasion of peritoneal reflection (mrT4a) and adjacent organs or structures (mrT4b) (2) (Figure 6).
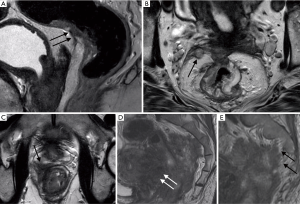 Figure 6 Rectal MRI in four patients: (A) Tumour extends into the peritoneal reflection. (B) mrEMVI involves the peritoneal reflection. (C) Tumour involves the right peripheral zone of the prostate gland. (D) Tumour invades the wall of the uterus and, in the same patient, (E) extends to the presacral fascia, involving the right S2 nerve root. These tumours require pelvic exenteration.
Figure 6 Rectal MRI in four patients: (A) Tumour extends into the peritoneal reflection. (B) mrEMVI involves the peritoneal reflection. (C) Tumour involves the right peripheral zone of the prostate gland. (D) Tumour invades the wall of the uterus and, in the same patient, (E) extends to the presacral fascia, involving the right S2 nerve root. These tumours require pelvic exenteration.
With every millimetre of extramural spread beyond 5 mm, the outcomes in terms of disease-free survival diminish. Defining the depth of invasion enables the large subgroup of mrT3 tumours to be stratified with greater prognostic accuracy. In a study by Merkel et al. involving 853 patients, mrT3 tumours with extramural spread of greater than 5 mm were associated with a 5-year cancer-specific patient survival rate of only 54% (26).
Patients with locally advanced mrT3 or mrT4 disease or with tumours threatening the potential circumferential resection margin on baseline MRI are offered chemoradiation therapy, which has been shown to reduce the tumour recurrence rate postoperatively (27).
MRI can predict the T stage with good accuracy (24). A systematic review and meta-analysis by Al-Sukhni et al. presented an accuracy of 85%, sensitivity of 87% and specificity of 75% of high-resolution rectal MRI in assessing rectal tumour T-category (13,28). The largest of the 21 studies included in this meta-analysis was the MERCURY study (25), which prospectively evaluated the accuracy of MR imaging in assessing the extramural depth of tumour invasion of rectal cancer compared to histopathologic results in 295 patients. The maximal extramural depth of tumour spread was measured, which is defined at histopathologic analysis by the distance from the outer edge of the longitudinal muscularis propria to the outer edge of the tumour. In 273 (92.5%) of the 295 patients, the depth of tumour spread reported on MR images was within 5 mm of the histopathologic measurement. The MR and histopathologic results were considered equivalent when the 95% confidence interval of the difference between them was within ±0.5 mm, suggesting that accurate measurements of extramural depth of tumour extension can be achieved on MRI (25).
Most staging failures with MRI occur in the differentiation of T2 and early T3 lesions (T3a), with over-staging as the major cause of errors. On MRI, it can be difficult to differentiate between spicules in the perirectal fat caused by fibrosis only, from spicules caused by fibrosis that contain tumour cells (29). The distinction between mrT2 stage and early mrT3 stage, however, is unlikely to be clinically significant because patients with early mrT3 lesions receive little benefit from preoperative neoadjuvant therapy and have similar prognosis to T1 and T2 tumours (4).
Review of images by consensus of two or more radiologists also increases accuracy (13,28). It is noted that in the MERCURY study, high resolution sequences were performed in 3 planes and participating gastrointestinal radiologists completed intensive training workshops, using correlated histopathologic and MR archives to ensure standardization of image acquisition techniques and interpretation of images (25).
Endorectal ultrasound (EUS) is accurate for staging superficial rectal tumours; however, it is limited in its utility in the staging of more advanced disease and evaluation of the mesorectal plane, limited by depth of acoustic penetration (4). Operator dependence, patient tolerance and lower accuracy for nodal staging compared to MRI are further disadvantages of this technique (4).
MRI depicts the morphology of the lesion, such as villous or polypoid. However, it does not reliably distinguish between benign and malignant lesions unless invasion is observed. A prospective study by Lee et al. evaluating resected lesions that were thought to be clinically benign determined that MRI correctly identified malignant polyps in only 44% of cases (30).
Circumferential resection margin (mrCRM)
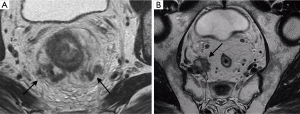
The CRM refers to the surgically dissected surface of the specimen that corresponds to the non-peritonealised portion of the rectum and along with the intersphincteric space can be thought of as the TME plane. The role of MR imaging is to alert the surgeon of a threatened mesorectal fascia (MRF) (24,31), which is defined by the tumour margin located within 1 mm of the MRF (Figure 7). The MRF and the CRM are not synonymous—MRF is defined anatomically, whereas CRM is determined by how the surgical procedure has been performed (16,24). MRI clearly demonstrates the MRF, which forms the circumferential resection margin at TME.
MR imaging is a consistent and reproducible technique with a high diagnostic accuracy (between 90% and 100%) for the evaluation of tumour invasion into the MRF and adjacent organs (2,5). It also has a high specificity (92%) for predicting a negative CRM (2). The accuracy in correctly predicting the CRM status is reduced with increasing proximity of the tumour to the anal verge. This may reflect difficulties in interpreting the closely opposed anatomical structures in this region (11,24).
The measurement of the distance of tumour to the mesorectal fascia on MRI preoperatively may distinguish between patients who will be cured by primary surgery and patients who are at high risk for locally invasive disease (2). A negative mrCRM (defined as 1 mm or more between the tumour edge/satellite deposits and the surgical margin) is associated with a significantly lower risk of a positive pCRM and local recurrence (4).
In a large prospective registry based study, Roodbeen et al. found five factors that increased the chance of pCRM after trans anal TME surgery: tumours within 1 cm from the anorectal junction, anterior tumours, mrT4 tumours, mrEMVI threatened and involved mrCRM on baseline imaging (21).
Extramural vascular invasion (mrEMVI)
EMVI is readily detected on MRI and is an important and independent prognostic feature (2). It is defined as tumour within the vessels extending beyond the muscularis propria (Figures 7 and 8).
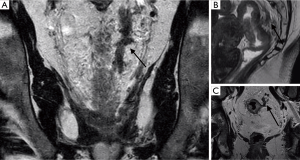
The presence and degree of extramural venous invasion predicts relapse-free survival—patients with advanced extramural venous invasion have a 3-year relapse-free survival rate of 35%, compared with 74% for patients with no or early extramural venous invasion (32). On MRI, EMVI is visualized as intermediate tumour signal intensity replacing the signal flow-voids that are normally seen in vessels on T2-weighted spin-echo sequences. The extramural vessels, which are oriented perpendicular to the rectal wall are also expanded (2). Lateral extension of EMVI can result in positive resection margins as vascular pathways do not respect the mesorectal fascia. EMVI and vascular tumour deposits close to the CRM pose a risk due to the potential for onward microscopic spread.
MR can predict EMVI with moderate sensitivity (62%) and relatively high specificity (88%) (32). Small vessels may be more difficult to assess (4).
Tumour deposits (mrTD)
These are thought to be discontinuous vascular tumour deposits in the mesorectal fat (Figure 7B). In a recent retrospective study, Lord et al. found that current MRI staging predicting nodal stage does not adequately predict prognosis. MRI detected tumour deposits have a greater prognostic accuracy. The presence of tumour deposits outranks nodal status and is a poor prognostic indicator. These patients should be treated more intensively and followed up more frequently due to higher risk of recurrence (33).
Lymph nodes
MR imaging evaluation of lymph nodes is limited (6). It has been suggested that approximately 25% of lymph nodes are over-staged (9), which potentially results in morbidity related to unnecessary preoperative chemoradiotherapy. There is evidence that in addition to limited accuracy of MRI, lymph node status on MRI does not have a significant impact on the patient’s prognosis and therefore this item may potentially be removed from the synoptic reports as more evidence emerges (33).
Measuring the size of lymph nodes is unreliable. Both metastatic lymph nodes and benign reactive nodes may be enlarged (2). Metastatic lymph nodes may also be small in size. No particular size cut-off is useful in predicting nodal status and 15% of metastatic lymph nodes measure less than 5 mm in short axis diameter (34). Despite this, measurement of node size is still included in many current guidelines (1).
Benign or reactive lymph nodes demonstrate uniform signal abnormality and smooth, sharply demarcated margins (4). Metastatic lymph nodes tend to demonstrate a nodular, irregular border and mixed signal intensity (4). A retrospective study by Kim et al. noted that lymph nodes with a mottled heterogenous pattern was associated with 50% sensitivity and 95% specificity for malignant involvement (35). The detection of spiculated or indistinct borders are associated with sensitivities of 45% and 36%, and specificities of 100% and 100%, respectively (2). Using the two criteria to diagnose involved lymph nodes, Brown et al. determined the sensitivity of MRI to be 85% (95% CI: 74%, 92%) and the specificity to be 97% (95% CI: 95%, 99%) (34). In the meta-analysis by Al-Sukhni et al., MRI performance was consistently poor for detection of lymph node metastases (28).
Although diffusion weighted imaging (DWI) is sensitive in nodal detection, it has no value for characterising lymph nodes, as there is significant overlap in ADC values for benign and malignant nodes (4). Therefore, DWI should not be used to assess lymph node status.
Good prognostic tumours, defined on MRI as ≤T3b stage without MRF involvement, have good outcomes, in terms of survival and local recurrence rates, irrespective of nodal stage (36). T3 tumours with 5 mm or less of extramural spread, were associated with a 5-year cancer-specific survival rate of greater than 85%, regardless of whether there was lymph node involvement (26).
Pathological lymph nodes that involve the CRM have been reported in only 1% to 2% of resected specimens (2). Lymph nodes rarely, if ever, cause a positive resection margin that results in a local recurrence. Extra-mesorectal lymph nodes, including pelvic side wall lymph nodes, are important to describe for treatment planning. Patients with involved pelvic sidewall nodes may undergo extended-field neoadjuvant radiotherapy. In addition, involvement of pelvic sidewall nodes may be a predictor of decreased overall survival and local recurrence (4).
Endorectal ultrasound is useful in predicting tumour depth, however it has limitations in the detection and characterisation of lymph nodes. A recent meta-analysis of 35 studies by Puli et al., which involved more than 2,700 patients, demonstrated a sensitivity of 73.2% and a specificity of 75.8% for EUS diagnosis of node involvement in rectal cancer (37). Therefore, the major role of EUS in rectal cancer staging is for assessment of tumour invasion depth, particularly in early-stage rectal tumours, for which EUS can be used to evaluate whether tumours are suitable for treatment by trans-anal or local excision (38). Accuracy of EUS for staging rectal cancer after radiation therapy is markedly reduced due to treatment-related oedema, inflammation, necrosis and fibrosis (39).
Primary or recurrent rectal cancer beyond TME planes
Beyond TME disease is defined as disease spread beyond the mesorectal fascia (40). This can be identified on high resolution MR images of the pelvis.
When reporting disease beyond the TME plane, the pelvis can be divided into 6 compartments according to the fascial boundaries and the anatomical planes of dissection between intrapelvic organs to help guide the surgical procedure (41). These are the items to be included in the synoptic report (17) (Appendix 2):
- Anterior peritoneal reflection at the level of the rectovesical pouch or rectouterine pouch of Douglas (Figure 6A,B). Involvement requires a peritonectomy and involvement of the compartment above the peritoneal reflection may require small bowel resection, sigmoid colectomy, ureterectomy, iliac vessel resection/reconstruction.
- Anterior compartment below the peritoneal reflection (Figure 6C,D). Involvement may require prostatectomy, hysterectomy, vaginal wall resection and reconstruction, cystectomy or urethrectomy.
- Posterior compartment (Figure 6E). Involvement of the presacral fascia, bony cortex/periosteum and the sacral segment may require coccygectomy or sacrectomy. Sciatic nerve or S1/S2 nerve root involvement can be assessed on MRI and impacts on the choice of surgery.
- Lateral compartment. This contains the ureters, external and internal iliac vessels, lateral pelvic lymph nodes, sciatic nerve, sciatic notch, S1 and S2 nerve roots, the piriformis and obturator internus muscles (Figure 7B and Figure 9). Involvement of these structures may require ureterectomy, iliac vessel resection/reconstruction, pelvic sidewall lymphadenectomy to achieve R0. Pelvic side wall infiltration is associated with a higher risk of systemic recurrence (42).
- The infra-levator compartment. This compartment contains the levator ani muscle, external sphincter complex and the ischioanal fossa (Figure 2). Abdominoperineal resection is required to achieve R0.
- Anterior urogenital triangle/perineum. For low rectal tumours, it is important to describe involvement of the vaginal introitus, urethra and retropubic space.
Georgiou et al. performed a retrospective assessment of 63 consecutive patients who underwent preoperative MRI planning prior to exenterative surgery for beyond TME disease (41). MRI had a sensitivity of ≥93.3% for all compartments, except the lateral compartment (89.3%). MRI specificity was lower in the posterior and anterior compartments (82% and 86.6%, respectively) compared to the other compartments (>93.5%) (41). Regardless of whether beyond TME disease is diagnosed at initial staging or in the setting of rectal tumour recurrence, resection margin status is the key prognostic indicator for long term outcome in patients who undergo pelvic exenteration for beyond TME plane disease (43). These patients can have good outcomes giving them the opportunity for long term survival and cure (42). Patients with pelvic side wall infiltration have an increased risk of positive margins and poorer long-term outcome (2).
Restaging post neoadjuvant chemoradiotherapy
For patients with locally advanced rectal cancer, neoadjuvant CRT improves local control, resulting in tumour downstaging in approximately 50% of patients and a pathologic complete response in 15–38% of cases. This may allow sphincter-preserving surgery to be performed or, in selected patients, may even offer a “watch and wait” non-surgical treatment approach (6).
Studies evaluating the diagnostic performance of MR in the restaging of locally advanced rectal cancer after neoadjuvant treatment have demonstrated variable results regarding tumour, nodal staging and tumour-free circumferential resection margin (CRM) evaluation.
A systematic review and meta-analysis by van der Paardt et al. indicated that MRI restaging of rectal cancer after preoperative chemoradiotherapy is challenging. Overall, ymrT stage showed a poor mean sensitivity (50.4%) and a good mean specificity (91.2%) (44).
Functional MR imaging shows promise in predicting tumour response to neoadjuvant chemoradiotherapy. DWI provides functional information of the microstructure of the tumour and low pre-treatment apparent diffusion coefficient (ADC) values might be associated with a more favourable response to chemotherapy and radiotherapy, however this requires further evaluation in randomly controlled trials (45). In addition, chemical shift MRI may potentially play a role in the future in predicting 5-FU resistant colorectal tumours (46).
The following items should be included in the post treatment synoptic report (Appendix 3):
Tumour regression grade (mrTRG)
MR imaging tumour regression grade assessment is based on principles similar to the pathologic TRG system, which examines the degree of tumour replacement by fibrotic stroma in the surgical specimens of rectal cancer post neoadjuvant therapy. The tumour is assessed to determine the proportion of fibrous tissue (low T2 signal) and tumour (intermediate T2 signal) (2). This is an important method for evaluating tumour response (2). It has been shown that patients with more fibrosis on post-neoadjuvant therapy specimens have improved survival relative to patients with less fibrosis, and that TRG is an independent predictor of overall and disease-free survival (4). Evaluating TRG on MRI cannot be considered as a reliable surrogate imaging marker of pathologic TRG in locally advanced rectal cancer patients who undergo neoadjuvant treatment (12). Nevertheless, TRG assessed on MRI is a potential tool for the implementation of treatment strategies following standard chemoradiotherapy (12).
T stage post treatment (ymrT)
As mentioned, the overall accuracy of MR imaging in restaging irradiated rectal cancers is much lower than initial staging MR imaging, with accuracies of approximately 50% for T stage. The main limitation in post-treatment MR assessment is differentiating fibrotic tissue containing tumor cells from fibrotic tissue without residual malignant cells (4). Assessment of the degree of mrTRG correlates with survival at a greater statistical significance than the mrT stage (5).
EMVI post-treatment (ymrEMVI)
EMVI may disappear after treatment or it may be replaced by fibrotic tissue, which may signify a good response to treatment (12). Regression of mrEMVI following neoadjuvant chemoradiotherapy results in improved patient outcomes. A retrospective study by Chand et al. demonstrated that fibrosis of mrEMVI of greater than 50% was associated with improved disease-free survival (47).
Lymph nodes post-treatment (ymrLN)
After chemoradiotherapy, MRI evaluation of lymph nodes remains challenging (6). It has been observed that after chemoradiotherapy, most involved lymph nodes become smaller and many disappear (48). Assessing the size of lymph nodes in the short axis may be more reliable than observing lymph node margin and shape to assess for residual malignancy in the post treatment setting (6). A meta-analysis by van der Paardt et al. concluded that MR imaging is not able to discriminate lymph node response after chemoradiotherapy (25).
CRM post treatment (ymrCRM)
MR imaging showed moderate accuracy for CRM staging with sensitivity of 76.3% and specificity of 85.9% (44).
For low rectal tumours, depth of invasion, involvement of the intersphincteric plane and external sphincter determine whether ultra low TME or intersphincteric APE can be safely performed or if ELAPE is required.
The role of imaging in local recurrence of rectal cancer
The overall local recurrence rate following treatment of rectal cancer is between 4-8% (6,49). High resolution MRI is superior to CT for diagnosis of local recurrence of rectal cancer (2,50,51); however, it may be difficult on both MRI and PET to diagnose recurrent rectal cancer due to overlap in the imaging appearances of recurrent disease and post treatment change on both modalities (4,51). Short interval follow-up MRI may confirm increase in size, invasion of adjacent structures and soft tissue asymmetry compared to a baseline study, suggesting tumour recurrence. T2 hyperintense signal on MRI and delayed contrast enhancement are not specific for differentiating between benign scar tissue, granulation tissue, haematoma and post radiation change (52). Serial serum carcinoembryonic antigen (CEA) measurements are also useful (53).
Patients with local recurrence should be referred to a specialist multidisciplinary team for diagnosis, assessment and elaboration of a treatment plan (50). Pelvic MRI plays a role in selecting patients in whom complete surgical excision with pelvic exenteration is possible and likely to improve long term survival and local control (43,54), such as in cases where the tumour recurrence is confined to the anastomotic site or in an anterior location in the pelvis. Surgery is less likely to achieve a pathological complete resection in lateral pelvic side wall recurrence (42,55). Following exenteration for beyond TME recurrence, there is a higher rate of positive resection margin when compared to patients with beyond TME primary tumour (43).
If pelvic exenteration is being considered, MRI is used to assess the extent of disease, invasion of proximal sacrum and lumbar spine, involvement of the lumbosacral plexus and sciatic nerves and encasement of the external or common iliac vessels (4,7). The presence of unresectable distant metastases should be determined with computed tomography of the chest and abdomen (4) which would be a contraindication to pelvic exenteration (56).
Conclusion
MRI plays a crucial role in the local staging of rectal cancer and guiding treatment decisions. It is a non-invasive and accurate tool for T stage assessment, involvement of the circumferential resection margin and extramural venous invasion. MRI is also useful in evaluation of rectal cancer that has extended beyond the TME planes, for post treatment evaluation and local recurrence, particularly for preoperative surgical planning. As greater validation of data and new research comes to light, such as the increasing importance of tumour deposits and decreasing relevance of lymph nodes as prognostic indicators, synoptic templates are continually updated. The use of a synoptic report is strongly encouraged as it ensures inclusion of all staging items—it is a dynamic document that is designed to reflect current evidence, promote efficient decision making and facilitate optimal patient care.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Eva Segelov) for the series “Colorectal Cancer” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/dmr-20-147
Peer Review File: Available at http://dx.doi.org/10.21037/dmr-20-147
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-20-147). The series “Colorectal Cancer” was commissioned by the editorial office without any funding or sponsorship. Dr. Brown reports grants from NIHR Royal Marsden BRC, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Beets-Tan RG. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2018;28:1465-75. [Crossref] [PubMed]
- Costa-Silva L, Brown G. Magnetic resonance imaging of rectal cancer. Magn Reson Imaging Clin N Am 2013;21:385-408. [Crossref] [PubMed]
- Moran B, Brown G, Cunningham D, et al. Clarifying the TNM staging of rectal cancer in the context of modern imaging and neo-adjuvant treatment: 'y''u' and 'p' need 'mr' and 'ct'. Colorectal Dis 2008;10:242-3. [Crossref] [PubMed]
- Furey E, Jhaveri KS. Magnetic resonance imaging in rectal cancer. Magn Reson Imaging Clin N Am 2014;22:165-90. v-vi. [Crossref] [PubMed]
- Patel UB, Taylor F, Blomqvist L, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011;29:3753-60. [Crossref] [PubMed]
- Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, et al. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics 2019;39:367-87. [Crossref] [PubMed]
- Taylor FG, Swift RI, Blomqvist L, et al. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol 2008;191:1827-35. [Crossref] [PubMed]
- García-Figueiras R, Baleato-González S, Padhani AR, et al. Advanced imaging of colorectal cancer: From anatomy to molecular imaging. Insights Imaging 2016;7:285-309. [Crossref] [PubMed]
- Bipat S, Glas AS, Slors FJ, et al. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology 2004;232:773-83. [Crossref] [PubMed]
- Heo SH, Kim JW, Shin SS, et al. Multimodal imaging evaluation in staging of rectal cancer. World J Gastroenterol 2014;20:4244-55. [Crossref] [PubMed]
- Taylor F, Mangat N, Swift RI, et al. Proforma-based reporting in rectal cancer. Cancer Imaging 2010;10 Spec no A:S142-50.
- Nougaret S, Jhaveri K, Kassam Z, et al. Rectal cancer MR staging: pearls and pitfalls at baseline examination. Abdom Radiol (NY) 2019;44:3536-48. [Crossref] [PubMed]
- Nougaret S, Reinhold C, Mikhael HW, et al. The use of MR imaging in treatment planning for patients with rectal carcinoma: have you checked the "DISTANCE"? Radiology 2013;268:330-44. [Crossref] [PubMed]
- Burton S, Brown G, Daniels IR, et al. MRI directed multidisciplinary team preoperative treatment strategy: the way to eliminate positive circumferential margins? Br J Cancer 2006;94:351-7. [Crossref] [PubMed]
- KSAR Study Group for Rectal Cancer. Essential Items for Structured Reporting of Rectal Cancer MRI: 2016 Consensus Recommendation from the Korean Society of Abdominal Radiology. Korean J Radiol 2017;18:132-51. [Crossref] [PubMed]
- Patel A, Rockall A, Guthrie A, et al. Can the completeness of radiological cancer staging reports be improved using proforma reporting? A prospective multicentre non-blinded interventional study across 21 centres in the UK. BMJ Open 2018;8:e018499. [Crossref] [PubMed]
- Siddiqui MRS, Shanmuganandan AP, Rasheed S, et al. An audit comparing the reporting of staging MRI scans for rectal cancer with the London Cancer Alliance (LCA) guidelines. Eur J Surg Oncol 2017;43:2093-2104. [Crossref] [PubMed]
- Nörenberg D, Sommer WH, Thasler W, et al. Structured Reporting of Rectal Magnetic Resonance Imaging in Suspected Primary Rectal Cancer: Potential Benefits for Surgical Planning and Interdisciplinary Communication. Invest Radiol 2017;52:232-9. [Crossref] [PubMed]
- Al-Sukhni E, Messenger DE, Charles Victor J, et al. Do MRI reports contain adequate preoperative staging information for end users to make appropriate treatment decisions for rectal cancer? Ann Surg Oncol 2013;20:1148-55. [Crossref] [PubMed]
- Pedersen BG, Blomqvist L, Brown G, et al. Postgraduate multidisciplinary development program: impact on the interpretation of pelvic MRI in patients with rectal cancer: a clinical audit in West Denmark. Dis Colon Rectum 2011;54:328-34. [Crossref] [PubMed]
- Roodbeen SX, de Lacy FB, van Dieren S, et al. International TaTME Registry Collaborative. Predictive factors and risk model for positive circumferential resection margin rate after transanal total mesorectal excision in 2653 patients with rectal cancer. Ann Surg 2019;270:884-91. [Crossref] [PubMed]
- Salerno G, Daniels IR, Moran BJ, et al. Clarifying margins in the multidisciplinary management of rectal cancer: the MERCURY experience. Clin Radiol 2006;61:916-23. [Crossref] [PubMed]
- Brown G, Kirkham A, Williams GT. High-resolution MRI of the anatomy important in total mesorectal excision of the rectum. AJR Am J Roentgenol 2004;182:431-9. [Crossref] [PubMed]
- Shihab OC, Moran BJ, Heald RJ, et al. MRI staging of low rectal cancer. Eur Radiol 2009;19:643-50. [Crossref] [PubMed]
- Mercury Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology 2007;243:132-9. [Crossref] [PubMed]
- Merkel S, Mansmann U, Siassi M, et al. The prognostic inhomogeneity in pT3 rectal carcinomas. Int J Colorectal Dis 2001;16:298-304. [Crossref] [PubMed]
- Patel UB, Blomqvist LK, Taylor F, et al. MRI after treatment of locally advanced rectal cancer: how to report tumor response--the MERCURY experience. AJR Am J Roentgenol 2012;199:W486-95. [Crossref] [PubMed]
- Al-Sukhni E, Milot L, Fruitman M, et al. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol 2012;19:2212-23. [Crossref] [PubMed]
- Beets-Tan RG. MRI in rectal cancer: the T stage and circumferential resection margin. Colorectal Dis 2003;5:392-5. [Crossref] [PubMed]
- Lee L, Arbel L, Albert MR, et al. Radiologic Evaluation of Clinically Benign Rectal Neoplasms May Not Be Necessary Before Local Excision. Dis Colon Rectum 2018;61:1163-9. [Crossref] [PubMed]
- Torkzad MR, Kamel I, Halappa VG, et al. Magnetic resonance imaging of rectal and anal cancer. Magn Reson Imaging Clin N Am 2014;22:85-112. [Crossref] [PubMed]
- Smith NJ, Barbachano Y, Norman AR, et al. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg 2008;95:229-36. [Crossref] [PubMed]
- Lord AC. MRI-diagnosed tumour deposits and EMVI status have superior prognostic accuracy to current clinical TNM staging in rectal cancer. Ann Surg 2020; Epub ahead of print. [Crossref] [PubMed]
- Brown G, Richards CJ, Bourne MW, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 2003;227:371-7. [Crossref] [PubMed]
- Kim JH, Beets GL, Kim MJ, et al. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol 2004;52:78-83. [Crossref] [PubMed]
- Taylor FG, Quirke P, Heald RJ, et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg 2011;253:711-9. [Crossref] [PubMed]
- Puli SR, Reddy JB, Bechtold ML, et al. Accuracy of endoscopic ultrasound to diagnose nodal invasion by rectal cancers: a meta-analysis and systematic review. Ann Surg Oncol 2009;16:1255-65. [Crossref] [PubMed]
- Kav T, Bayraktar Y. How useful is rectal endosonography in the staging of rectal cancer? World J Gastroenterol 2010;16:691-7. [Crossref] [PubMed]
- Siddiqui AA, Fayiga Y, Huerta S. The role of endoscopic ultrasound in the evaluation of rectal cancer. Int Semin Surg Oncol 2006;3:36. [Crossref] [PubMed]
- Havenga K, Grossmann I, DeRuiter M, et al. Definition of total mesorectal excision, including the perineal phase: technical considerations. Dig Dis 2007;25:44-50. [Crossref] [PubMed]
- Georgiou PA, Tekkis PP, Constantinides VA. Diagnostic accuracy and value of magnetic resonance imaging (MRI) in planning exenterative pelvic surgery for advanced colorectal cancer. Eur J Cancer 2013;49:72-81. [Crossref] [PubMed]
- Mariathasan AB, Boye K, Giercksky KE, et al. Beyond total mesorectal excision in locally advanced rectal cancer with organ or pelvic side-wall involvement. Eur J Surg Oncol 2018;44:1226-32. [Crossref] [PubMed]
- Bhangu A, Ali SM, Brown G, et al. Indications and outcome of pelvic exenteration for locally advanced primary and recurrent rectal cancer. Ann Surg 2014;259:315-22. [Crossref] [PubMed]
- van der Paardt MP, Zagers MB, Beets-Tan RG, et al. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology 2013;269:101-12. [Crossref] [PubMed]
- Amodeo S, Rosman AS, Desiato V, et al. MRI-Based Apparent Diffusion Coefficient for Predicting Pathologic Response of Rectal Cancer After Neoadjuvant Therapy: Systematic Review and Meta-Analysis. AJR Am J Roentgenol 2018;211:W205-W216. [Crossref] [PubMed]
- Shepelytskyi Y, Fox MS, Davenport K, et al. In-Vivo Retention of 5-Fluorouracil Using 19F Magnetic Resonance Chemical Shift Imaging in Colorectal Cancer in a Murine Model. Sci Rep 2019;9:13244. [Crossref] [PubMed]
- Chand M, Swift RI, Tekkis PP, et al. Extramural venous invasion is a potential imaging predictive biomarker of neoadjuvant treatment in rectal cancer. Br J Cancer 2014;110:19-25. [Crossref] [PubMed]
- Heijnen LA, Maas M, Beets-Tan RG, et al. Nodal staging in rectal cancer: why is restaging after chemoradiation more accurate than primary nodal staging? Int J Colorectal Dis 2016;31:1157-62. [Crossref] [PubMed]
- Tan WJ, Tan HJ, Dorajoo SR, et al. Rectal Cancer Surveillance-Recurrence Patterns and Survival Outcomes from a Cohort Followed up Beyond 10 Years. J Gastrointest Cancer 2018;49:422-8. [Crossref] [PubMed]
- Beyond TME. Collaborative. Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br J Surg 2013;100:E1-33. [Crossref] [PubMed]
- Krestin GP, Steinbrich W, Friedmann G. Recurrent rectal cancer: diagnosis with MR imaging versus CT. Radiology 1988;168:307-11. [Crossref] [PubMed]
- Dicle O, Obuz F, Cakmakci H. Differentiation of recurrent rectal cancer and scarring with dynamic MR imaging. Br J Radiol 1999;72:1155-9. [Crossref] [PubMed]
- Grossmann I, de Bock GH, Meershoek-Klein Kranenbarg WM, et al. Carcinoembryonic antigen (CEA) measurement during follow-up for rectal carcinoma is useful even if normal levels exist before surgery. A retrospective study of CEA values in the TME trial. Eur J Surg Oncol 2007;33:183-7. [Crossref] [PubMed]
- Dresen RC, Kusters M, Daniels-Gooszen AW, et al. Absence of tumor invasion into pelvic structures in locally recurrent rectal cancer: prediction with preoperative MR imaging. Radiology 2010;256:143-50. [Crossref] [PubMed]
- Moore HG, Shoup M, Riedel E, et al. Colorectal cancer pelvic recurrences: determinants of resectability. Dis Colon Rectum 2004;47:1599-606. [Crossref] [PubMed]
- Sagebiel TL, Viswanathan C, Patnana M, et al. Overview of the Role of Imaging in Pelvic Exenteration. Radiographics 2015;35:1286-94. [Crossref] [PubMed]
Cite this article as: Hui CL, Riazi Y, Mautone M, Brown G. Role of imaging and synoptic MRI reporting in determining optimal management paradigm for rectal cancer: a narrative review. Dig Med Res 2020;3:47.







