
Infection control strategy for COVID-19 at endoscopy unit in the era of COVID-19 outbreak
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) related disease, known as coronavirus disease 2019 (COVID-19), has spread world over and has been declared a global pandemic by the World Health Organization (WHO) on 11th March, 2020. COVID-19 not only causes respiratory symptoms such as cough, fever, sputum production, and shortness of breath, but may also produce gastrointestinal symptoms including nausea, vomiting, and diarrhea (1). The rate of severe COVID-19 is 13.9%, with an overall mortality rate of 2.3% (2). SARS-CoV-2 is mainly transmitted through aerosols, droplets, and direct contact (3,4). Performing upper endoscopic procedures such as esophagogastroduodenoscopy (EGD), endoscopic ultrasonography (EUS), and endoscopic retrograde cholangiopancreatography (ERCP), can predispose endoscopists and medical staff to infection as aerosols may be generated by patients coughing, retching, or belching during the procedure. Furthermore, performing colonoscopy (CS) may also increase the risk of SARS-CoV-2 transmission as it has been detected in the fecal samples of infected subjects (5). Currently, some international guidance recommends essential steps, such as stringent patient triage (prescreening), endoscopic procedure stratification, and appropriate use of personal protective equipment (PPE) to prevent peri-procedural transmission of SARS-CoV-2 of endoscopic personnel and nosocomial infection between patients (6-10). We are conducting an infection control strategy (ICS) of our endoscopy unit according to those guidance, especially statements of the Asian Pacific Society for Digestive Endoscopy (APSDE) that greatly influenced our ICS (6). In addition, we have carried out several characteristic ICS in our endoscopy unit. This article aim is to summarize the ICS of APSDE for COVID-19 and to present the characteristic ICS of our endoscopy unit based on these statements.
Prescreening
Prescreening is a basic prerequisite in endoscopic unit workflow management and the ICS (7,9,11). First, at the stage of patient triage, subjects should undergo prescreening for symptoms and epidemiological history, such as fever, travel history to the pandemic area, occupation, cluster of cases, and contact of a suspected or confirmed case (FTOCC). The APSDE recommended patients to apply to any of the positive criteria for FTOCC should be regarded as suspected cases (6). In addition, a chest computerized tomography (CT) should be performed prior to endoscopic procedures during the COVID-19 pandemic. Subjects responding affirmatively to any one of the above questions or demonstrating an abnormality on the chest CT should be regarded as suspected cases, and as many of them as possible must undergo polymerase chain reaction (PCR) testing for SARS-CoV-2. Patients whose PCR test for SARS-CoV-2 is positive should be considered as confirmed COVID-19 cases, and they must not undergo endoscopic procedures in the absence of urgent indications. Suspected cases who could not receive the PCR test for SARS-CoV-2 mandate the same amount of precautionary measures as confirmed COVID-19 cases. Considering asymptomatic cases with COVID-19 (2), if possible, all patients might have to receive a PCR test before endoscopic procedures (12). Patients with no history of FTOCC, a normal chest CT scan, and/or with PCR test negative for SARS-CoV-2 are regarded as being at low risk. However, endoscopic procedures for low-risk cases without emergency or life-threatening conditions should be postponed during the COVID-19 pandemic because of insufficient sensitivity of the PCR test for SARS-CoV-2 (13,14).
Endoscopic procedure stratification according to urgency
For endoscopic procedure stratification, endoscopists should categorize indications according to level of urgency as: urgent, semi-urgent, and elective cases (6). Urgent indications, such as acute gastrointestinal bleeding, acute obstructive suppurative cholangitis, foreign body in the esophagus, and stenting for gastrointestinal obstruction, require endoscopy to be promptly performed. Semi-urgent endoscopic procedures include diagnosis and/or treatment of malignancies. To determine whether the semi-urgent endoscopic procedure needs to be performed or may be postponed, a case-by-case evaluation is needed. Elective procedures such as routine diagnostic, surveillance, and follow-up endoscopy, can be postponed until the COVID-19 pandemic is well controlled.
As a measure against the spread of COVID-19, The Government of Japan had a declaration of a state of emergency in all Japan between April 16th, 2020, and May 25th, 2020. In our hospital, some elective endoscopic procedures were also postponed during a state of emergency over COVID-19. However, in Tottori prefecture, only a patient was infected with COVID-19 on April 16th, 2020, so that all the elective cases were not postponed. Indeed, 739 of elective procedures, including 450 of EGD, 199 of CS, 55 of EUS, 27 of ERCP, and 8 of capsule enteroscopy were performed in our hospital between April and May 2020. Compared to the same period in 2019, 81% of the elective procedures were performed. Meanwhile, 78 of urgent procedures (Acute gastrointestinal bleeding, 64; Foreign body in esophagus, 2; ERCP for acute cholangitis, 9; Stenting for gastrointestinal obstruction, 3) and 186 of semi-urgent procedures (Endoscopic submucosal dissection/Endoscopic mucosal resection for gastrointestinal cancer, 32; EGD and CS for a highly suspicious case of malignant disease, 70; ERCP for a highly suspicious case of hepatobiliary/pancreas cancer, 37; EUS-guided fine needle aspiration for a highly suspicious case of hepatobiliary/pancreas cancer, 21; Small bowel enteroscopy for occult gastrointestinal bleeding, 2) were undergone between April and May 2020. Compared to the same period in 2019, 105% of the urgent procedures and 126% of the semi-urgent procedures were performed. Although 3 patients were infected with COVID-19 in Tottori prefecture between April and May 2020, there was no peri-procedural SARS-CoV-2 transmission in our endoscopy unit.
PPE
All healthcare workers working at an endoscopy unit should know the appropriate use of PPE during an endoscopic procedure (6). Medical supplies, including PPE, will be limited during the pandemic because of insufficient supply during the pandemic so that the use of PPE should be optimized according to each patient’s risk for SARS-CoV-2 infection. Standard PPE, including a surgical mask, gloves, isolation gown with water resistance, hairnet or headcovers, and protective eyewear (goggles or face shield), should be used for low-risk cases during peri-endoscopy. For confirmed, suspected cases of COVID-19, enhanced PPE, including N-95 or FFP-2/3 high-filter respiratory masks, two pairs of gloves, isolation gown with water resistance, hairnets or headcovers, protective eyewear (goggles or face shield), and shoe covers should be used during endoscopy.
We develop a novel face-protective shield, called as ORIGAMI (Japan Article Number code 4580107634378, Medi Beat Inc.), and use that as protective eyewear in our hospital (15). The ORIGAMI is composed of coated cardboard and the polypropylene film with lightweight (approximately 31 g).
The Video 1 shows how to make the ORIGAMI. The ORIGAMI can be constructed easily and it is almost 1 minutes that the entire process takes. During making ORIGAMI, don’t touch the clear film. We evaluated the utility of ORIGAMI to protect the face from aerosols. By wearing ORIGAMI on a doll’s face, the protective ability of ORIGAMI was evaluated. A doll with ORIGAMI was dressed in a surgical mask, hairnet, and ORIGAMI. The other doll without ORIGAMI was dressed in a surgical mask and hairnet. To imitate the patient’s cough or retching, experimentally, a balloon with a 7-inch size containing 5×103 L of indigo carmine was inflated with air and ruptured with a needle device. The distance between the dolls’ faces and the balloon was 25 cm. After 3 balloons were ruptured, the stains of dolls’ faces, surgical masks, hairnets were compared respectively. Some stains appeared in the face, the surgical mask, and the hairnet of the doll without ORIGAMI. Some stains also appeared in the surface of the ORIGAMI. However, there was no stain in the face and the surgical mask of the doll with ORIGAMI.
Furthermore, the ORIGAMI can be expected to prevent the surgical mask and N-95 respiratory mask, which are limited during the COVID-19 pandemic, from contaminations caused by droplets and aerosols. The film is less cloudy because of the distance between the face and the shield film. Unlike goggles, the ORIGAMI, made at a cost of approximately $1, can be used as a disposable. The ORIGAMI can help the reduction of risk of SARS-CoV-2 transmission to the healthcare workers during peri-endoscopy and preserve the PPE resources.
Workflow of endoscopy unit
During the era of COVID-19 outbreak, an endoscopy unit should not only educate healthcare workers on peri-procedural protection from transmission of SARS-CoV-2 but also establish an optimally conducive workflow and workspace (7,16). In the future, we need to be prepared for any unknown as well as known, resistant infectious agents that spread via airborne transmission. Therefore, it is necessary to organize the workflow and workspace as effective countermeasures in endoscopy units in tertiary-care and referral hospitals.
The endoscopy unit should be separated into three zones: contaminated, clean, and buffer zones (7,17,18). The route of healthcare workers, including the entrance and exit of the endoscopy unit, should be isolated from the patients’ circulation lines expect procedure rooms (19). At the outpatients’ entrance, the non-contact thermometer screening is positioned, followed by the patient triage (prescreening) at the triage room where symptoms and epidemiological history, including FTOCC, can be reviewed mechanically by using a mobile tablet or similar device. In places, including reception and triage room, where prescreening for patients’ stratification is performed, separation in space or time among each case is required. In our hospital, there is a patient call system “TORIRINRIN” calling for patients by mobile phone using application software that has been developed inhouse (Figure 1). Our patients can download and get the free app into their mobile phones whenever anyone wishes. The institutional voluntary receptionist clerks support the app maneuver for helping patients who are not familiar with such mobile operations, such as the elderly. First, patients can accept the reception using their mobile phones while they are within the radius of the hospital 500 meters. Next, they can wait in places where people are not crowded, such as in their cars. Notice is given to the patients about 15 minutes before the examination, and then they wait near the endoscopy unit, such as a waiting room in an endoscopy unit. After the call, patients come into the endoscopic procedure rooms. The statements of APSDE described it is important to keep all patients at an appropriate distance from each other in the endoscopy unit (6). This system could prevent the patient from being crowded in the reception and waiting room. Soon, we would like to develop a new system to prescreen any suspicious symptoms and epidemiological history, including FTOCC, cough, shortness of breath, diarrhea, dysgeusia, and dysosmia with no other etiology. This system could not only perform triage of patients before they enter the hospital using mobile phones but also avoid situations of called 3 C, including closed spaces, crowds, and close contact. It is considered an essential step of ICS for COVID-19.
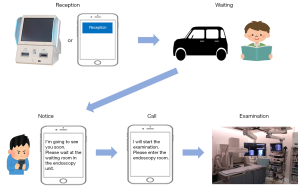
After patients are stratified into groups of suspected COVID-19 cases and low-risk cases by this prescreening, a circulation line for patients suspected of COVID-19 needs to separate, and completely isolate those patients from COVD-19 low-risk patients.
Then, the suspected cases receive the PCR test at the single preparation room (contaminated zones). Negative pressure and air exchange systems are required in the contaminated zone. The high-efficiency particulate air filtration equipment can be instead of negative pressure settings. In confirmed COVID-19 cases by the PCR test, the endoscopic procedures should be indicated in only emergency cases, and should be performed in a negative pressure room (contaminated zones). Similarly, if an urgent endoscopic procedure is indicated in suspected COVID-19 cases who have not been administered the PCR test, it must be performed in a negative pressure room.
Among low-risk patients with PCR test negative for SARS-CoV-2, it is not necessary to perform endoscopic procedures in a negative pressure room, and endoscopy in such patients may be performed in the clean zones.
It is enough for healthcare workers to use standard PPE in clean zones. Meanwhile, enhanced PPE should be used by healthcare workers in contaminated zones. Before entering the contaminated zones, the healthcare workers need to wear or remove enhanced PPE in the changing rooms (buffer zones). Although almost contaminated PPE should be removed in the procedure room (contaminated zone), the N-95 respiratory masks should be removed in the changing room (20). Furthermore, the changing room needs to be separated into wearing and removing areas to prevent cross-contamination by means of at least a partition. For the transportation of contaminated equipment (including endoscopes), the one-way passages are needed to avoid cross-contamination with disinfected equipment (7,17). Therefore, automated endoscope reprocessors must be installed both in the clean zones and in the contaminated zones. The disinfection and reprocessing of the contaminated equipment used for a confirmed/suspected COVID-19 case will be similar to standard practice. If an endoscopic procedure was undergone for confirmed COVID-19 case in a procedure room, the next case should be due after at least 30 minutes for full decontamination (6).
How to resume elective endoscopic procedures
To resume elective endoscopic procedures, we should consider the number of newly infected patients with COVID-19 and the availability of medical supplies, including PPE (6). The statements of APSDE recommended that the endoscopy unit resumes an elective endoscopy service in a stepwise manner according to the prevalence of COVID-19. We could consider resuming elective endoscopic procedures gradually when the downtrend in the new patients with COVID-19 and the suboptimal medical supplies reserve, which may be between 4 and 8 weeks. If there are no newly developed patients with COVID-19 at least the last 2 weeks, and the normal amount of medical supplies’ storage in the endoscopy unit, the elective endoscopic procedures could be fully resumed.
After cancellation of the emergency declaration for COVID-19, during June 2020, 468 of elective procedures, including 336 of EGD, 81 of CS, 36 of EUS, 11 of ERCP, and 4 of capsule enteroscopy, were performed in our hospital. Therefore, compared during a state of emergency over COVID-19, 127% of elective procedures performed on June 2020. Fortunately, there was no problem with the availability of medical supplies in our hospital so that we could resume the endoscopic procedures for elective cases. However, the number of newly infected patients with COVID-19 per day has been increasing since July 2020, reaching 1605 on August 7 in Japan. Without the declaration a state of emergency over COVID-19, the endoscopy unit should manage the number of endoscopic procedures considering the number of newly developed COVID-19 patients and the availability of medical supplies.
In conclusion, gastrointestinal endoscopists and healthcare workers are at high risk for SARS-CoV-2 infection in this era of the COVID-19 outbreak. Endoscopy units should manage ICS, including stringent patient triage (prescreening), endoscopic procedure stratification, appropriate use of PPE, the workflow, and the management of the number of endoscopic procedures to prevent and minimize the risk of peri-procedural transmission of SARS-CoV-2.
Acknowledgments
This work was supported by our colleagues in the Division of Gastroenterology and Nephrology, Department of Multidisciplinary Internal Medicine, Tottori University Faculty of Medicine (Tottori, Japan). The authors are grateful to Prof. Masaru Ueki (Division of Medical Education, Department of Medical Education, Faculty of Medicine, Tottori University), Atsuro Koga (Research Strategy Division, Organization for Research Initiative and Promotion, Tottori University), Kazutake Uehara (Advanced Medicine, Innovation and Clinical Research Center, Tottori University Hospital) for help to perform our experiment.
Funding: None.
Footnote
Provenance and Peer Review: This article was a free submission to the journal. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-20-122). Hajime Isomoto serves as an unpaid editorial board member of Digestive Medicine Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708-20. [Crossref] [PubMed]
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China. JAMA 2020;323:1239-42. [Crossref] [PubMed]
- Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N Engl J Med 2020;382:1199-207. [Crossref] [PubMed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- Cheung KS, Hung IF, Chan PPY, et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 2020;159:81-95. [Crossref] [PubMed]
- Chiu PWY, Ng SC, Inoue H, et al. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements). Gut 2020;69:991-6. [Crossref] [PubMed]
- Lui RN, Wong SH, Sánchez-Luna SA, et al. Overview of guidance for endoscopy during the coronavirus disease 2019 pandemic. J Gastroenterol Hepatol 2020;35:749-59. [Crossref] [PubMed]
- American Society for Gastrointestinal Endoscopy. guidance for trainees during the COVID-19 pandemic. Gastrointest Endosc 2020;92:748-53. [Crossref] [PubMed]
- Castro Filho EC, Castro R, Fernandes FF, et al. Gastrointestinal endoscopy during the COVID-19 pandemic: an updated review of guidelines and statements from international and national societies. Gastrointest Endosc 2020;92:440-5. [Crossref] [PubMed]
- Irisawa A, Furuta T, Matsumoto T, et al. Gastrointestinal endoscopy in the era of the acute pandemic of coronavirus disease 2019: Recommendations by Japan Gastroenterological Endoscopy Society. Dig Endosc 2020;32:648-50. [Crossref] [PubMed]
- Guda NM, Emura F, Reddy DN, et al. Recommendations for the Operation of Endoscopy Centers in the setting of the COVID19 pandemic - A WEO guidance document. Dig Endosc 2020;32:844-50. [Crossref] [PubMed]
- Corral JE, Hoogenboom SA, Kröner PT, et al. COVID-19 polymerase chain reaction testing before endoscopy: an economic analysis. Gastrointest Endosc 2020;92:524-34.e6. [Crossref] [PubMed]
- Pan Y, Li X, Yang G, et al. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect 2020;81:e28-32. [Crossref] [PubMed]
- Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol 2020;92:1518-24. [Crossref] [PubMed]
- Onoyama T, Fuji M, Isomoto H. Useful face‐protective shield “ORIGAMI” for gastrointestinal endoscopy during the COVID‐19 pandemic. Dig Endosc 2020;32:998. [Crossref] [PubMed]
- Onoyama T, Isomoto H. A perspective gastrointestinal endoscopy infection control strategy against COVID-19: workflow and space management for the operation of endoscopic centers. Dig Endosc 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Das A. Impact of the COVID-19 pandemic on the workflow of an ambulatory endoscopy center: an assessment by discrete event simulation. Gastrointest Endosc 2020;92:914-24. [Crossref] [PubMed]
- Cennamo V, Bassi M, Landi S, et al. Redesign of a GI endoscopy unit during the COVID-19 emergency: A practical model. Dig Liver Dis 2020;52:1178-87. [Crossref] [PubMed]
- Zhang S, Wu X, Pan H, et al. Gastrointestinal Endoscopy Infection Control Strategy during COVID-19 Pandemic: experience from a tertiary medical center in China. Dig Endosc 2020; [Crossref] [PubMed]
- Onoyama T, Isomoto H. COVID-19 and gastrointestinal endoscopy: Importance of reducing SARS-CoV-2 infection risks of medical workers and preserving personal protective equipment resources. Dig Endosc 2020;32:732-5. [Crossref] [PubMed]
Cite this article as: Onoyama T, Teramoto K, Kurumi H, Fujii M, Isomoto H. Infection control strategy for COVID-19 at endoscopy unit in the era of COVID-19 outbreak. Dig Med Res 2020;3:34.


