Surgical stress response and cancer outcomes: a narrative review
Surgical stress response
Surgery is inherently a process of trauma, and one which generates significant metabolic and hormonal changes within the body (1). In response to any injury, there is activation of the sympathetic nervous system, endocrinological and immunological changes (2).
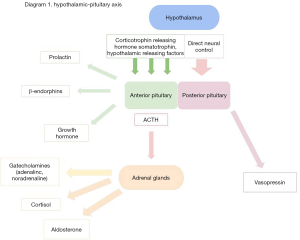
Activation of the sympathetic nervous system starts at the site of injury or trauma, with both somatic and autonomic afferent nerve activation of nociceptors within unmyelinated C fibres and myelinated A-δ fibres (3). These nerves exert effects upon the usual state of homeostasis with failure of negative feedback controlling hormone release within the hypothalamus and pituitary glands, leading to an increase in release of catabolic hormones like catecholamines and a decrease in anabolic hormones like insulin (4) (Table 1). Clinically, these changes will manifest as hypertension and tachycardia, but there will also be functional effects on the kidneys, liver and pancreas (Table 1) (3), in addition to the effects exerted by sympathetic activation of the hypothalamus and thus the hypothalamic-pituitary-adrenal axis. The hypothalamus controls release of pituitary hormones via release of inhibitory tone or hormone release (4).
Table 1
| Increased secretion | Effect | Decreased secretion | Effect | |
|---|---|---|---|---|
| Pituitary gland | Growth hormone (GH) | Promotes glycogenolysis and lipolysis | Testosterone | Clinical significance of reduced secretion is unclear, levels usually return to normal days after surgery |
| Adrenocorticotrophic hormone (ACTH) | Stimulates cortisol production | Oestrogen | ||
| β-Endorphin | Altered immune function | Triiodothyronine (T3) | ||
| Prolactin | Altered immune function | |||
| Vasopressin | Vasopressor effects, stimulates ACTH release, increased haemostasis | |||
| Adrenal gland | Catecholamines | Sympathetic nervous system activation; tachycardia, hypertension | ||
| Cortisol | Protein breakdown within skeletal muscle, hyperglycaemia, sodium and water retention, decreased inflammatory mediators | |||
| Aldosterone | Increased sodium retention | |||
| Pancreas | Glucagon | Glycogenolysis, mobilisation of free fatty acids (FFAs) | Insulin | Decreased glycogen synthesis, increased circulating glucose |
Stimulation of the pituitary gland results in an increase in catabolic hormone circulation and a decrease in anabolic hormones such as insulin and testosterone (Table 1, Figure 1). The end result is an increase in cortisol, with failure of negative feedback systems resulting in a sustained increase during and after surgery (4). Cortisol promotes gluconeogenesis and favours protein breakdown at a rate that can exceed synthesis. This can result in hyperglycaemia, which can impede wound healing and increase risk of postoperative infection (5), and skeletal muscle breakdown and postoperative muscle weakness. Cortisol also increases retention of sodium and water and decreases circulating inflammatory mediators such as prostaglandins and cytokines. This suppression of cellular immunity is another important response to surgical stress or trauma (6). Levels of cytokines, for example, interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-8 (IL-8) and tumour necrosis factor-alpha (TNF-ɑ), have been seen to increase in the immediate post-operative period following synthesis by macrophages, fibroblasts and endothelial cells in response to direct trauma and inflammatory stimuli (3). Cytokines mediate inflammation through local effects on target receptors, for example through stimulating the liver with subsequent synthesis and secretion of acute phase proteins like C-Reactive Protein (CRP), alpha-1 acid glycoprotein and immunosuppressive acidic protein (IAP) (7). The complex interaction of cortisol, acute phase reactants and cytokines leads to cellular immunosuppression and reduced natural killer (NK) cells. This has been shown to accelerate the growth of tumour cells and likelihood of metastatic spread (6).
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/dmr-20-94).
Surgical stress response and cancer
The primary cause of cancer mortality is metastasis (8), a multistage process whereby tumour cells spread from the primary growth to distant organs (9). In general, this process is the same for all solid tumours, and can theoretically be halted at any step. There are several distinct steps; development of a vascular network within the tumour, evasion of the host’s immune response and distant organ specific factors that increase tumour growth rate (8).
Development of a vascular network
Manipulation of tumour tissue and its blood supply have been shown to increase shedding of tumour cells into blood and lymphatics (10). Handling of tumour tissue during surgery can increase this risk and can be minimised with use of minimally invasive surgical techniques such as laparoscopic and robotic surgery. Neovascularisation enables tumours to enter systemic circulation and allows solid metastatic tumours to grow larger than 1 mm (11). This is generally a complex process that requires activation of signalling pathways to enable cell proliferation (12). Proteins that are known to promote angiogenesis include vascular endothelial growth factor (VEGF), interleukin-8 (IL-8), interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-ɑ), some of which are a cytokine end product of the stress response produced by surgical stimulation (11). Reduction of these angiogenesis-promoting hormones and the subsequent neovascularisation may be possible by reducing the surgical stress response, thereby reducing the chance of angiogenesis and likelihood of cancer spread. Catecholamines produced by the stress response, such as noradrenaline, have been shown to upregulate vascular endothelial growth factor (VEGF) through beta adrenergic receptor activation and cyclic AMP signalling pathways in adipose tissue, nasopharyngeal cancer cells and ovarian cancer cells in vitro, resulting in increased invasion potential, an effect that has the potential to be modulated through use of perioperative adreno-receptor blocking agents (13). The stress hormones adrenaline and noradrenaline have also been shown to increase the invasive potential of metastatic cells via increases in matrix metalloproteinase protein (MMP) production in ovarian cancer cells (12). These effects may be attenuated by reduction of the surgical stress response itself.
Invasion
Once tumour cells have entered the host’s microcirculation, they must circulate through the bloodstream to invade distant organs. Once they reach the organ of metastasis, tumour cells must form stable interactions with endothelial cell surfaces (14). Adhesion to vascular endothelium occurs in a similar way to leukocytes. Cancer cells stimulate production by cytokines of endothelial cell glycoproteins (E-Selectin) that usually direct neutrophils to damaged tissue but instead keep the metastatic cells within distant organs through the formation of adhesive ligand bonds (14). Blockade of E-Selectin within microcirculation has been shown to reduce the frequency of liver metastasis in some animal models (14). Once metastatic cells have been trapped within a distant organ’s microcirculation, growth occurs within the blood vessel or in tissue parenchyma.
Tumour growth
Currently there is a hypothesis of an ongoing process known as ‘immunoediting’, a process that may keep distant metastases at bay by preventing accelerated growth to clinically significant numbers of tumour cells (15). There are three phases to immunoediting; elimination, equilibrium and escape.
In elimination, innate and adaptive immunological factors such as natural killer (NK) cells detect and destroy tumour cells. There is evidence that surgical patients can have decreased levels of NK cells due to suppression by circulating prostaglandins, catecholamines and volatile anaesthetics (15).
If elimination fails, equilibrium occurs with adaptive immunological factors like IL-12, T cells and IFN-y preventing ongoing tumour growth. Tumour cells can develop further and no longer be recognised by adaptive factors, become insensitive to immune factors and/or local immunosuppression develops. If this happens the tumour can ‘escape’ and proliferate (16). It has been shown that dopamine and noradrenaline can trigger apoptosis in some cells, implying that the presence of these may be protective against tumour proliferation (17). However, there is evidence that in some prostate and breast cancer cells, adrenaline can protect against apoptosis through beta-2 adrenergic receptors and inactivation of the pro-apoptotic protein BCL2 agonist of cell death (BAD) (18). This suggests that circulating catecholamines like adrenaline as part of the surgical stress response may contribute to tumour growth and proliferation via prevention of apoptosis. Catecholamines also function synergistically with corticosteroids to facilitate growth of some cancers. Il-6 and growth factors such as vascular endothelial growth factor (VEGF) can promote cell growth and progression through activation of the signal transducer and activation of transcription-3 protein (STAT-3), which promotes angiogenesis (as discussed above) and also suppresses apoptosis, therefore contributing to tumour growth (12).
Perioperative modifications to improve cancer outcomes
Prehabilitation programmes are becoming increasingly popular before major surgery (19). In cancer patients there is a particular risk of deconditioning due to age-related co-morbidities, neo-adjuvant chemotherapy, inactivity and the direct effects of tumours (17). For example, oesophageal and oral tumours may impact on a patient's nutritional state due to inability to eat properly, lung tumours impair gas exchange and lead to decreased exercise tolerance and bleeding lower gastrointestinal tumours can result in iron-deficiency anaemia and deconditioning. Improving a patients’ physiology can have beneficial effects on their immune function, and there is evidence that exercise can reduce risk of cancer recurrence in patients with diagnosis of colon cancer by decreasing systemic levels of pro-inflammatory cytokines (20). Exercise has been shown to increase circulating NK and T cells following inflammation caused by shear stress in muscles and increased catecholamines (21). This increased recruitment, and redistribution of NK cells particularly, may lead to longer-term protection against tumour growth and metastasis. However, more evidence is needed in this area as available data is largely in vitro and has yet to be replicated on a wider scale in vivo (22).
Nutrition is another important consideration for cancer patients, who may be particularly at risk of being malnourished due to local and systemic tumour effects, chemotherapy and/or lifestyle. Supplementation of nutrition or aiding feeding via a gastric or jejunal feeding tube may aid in the prevention of perioperative infections, blood transfusion and decrease hospital length of stay (23).
Reducing the stress response is a key strategy for surgery in cancer patients, to reduce systemic inflammation and cytokine storm as detailed earlier.
Anaesthetic agents
There has been much recent research both in vitro and in vivo on the effects of commonly-used volatile anaesthetic agents (such as sevoflurane) on tumour growth and cancer outcomes (24). Inhalational anaesthetic agents are thought to promote angiogenesis and tumour growth via upregulation of hypoxia inducible factors (HIFs) (25). Hypoxia inducible factors are transcription factors that regulate cell adaptation to stress caused by hypoxia through angiogenesis (26). They are overexpressed in tumours and promote growth and proliferation. An in vitro study in 2015 exposing glioma stem cells to sevoflurane showed significant increases in HIF-1α and VEGF in a concentration dependent manner with evidence of increased cell proliferation (25). Isoflurane and desflurane have shown similar increases (27).
In contrast, Total Intravenous Anaesthesia (TIVA) with propofol and remifentanil theoretically suppresses angiogenesis. A two year follow up of patients with primary breast cancer showed reduced levels of vascular endothelial growth factor-C (VEGF-C) in patients who received TIVA compared with those who received maintenance of anaesthesia with inhaled sevoflurane (27).
Inhalational anaesthetics are also associated with a reduction in NK cells, via inhibition of adhesion molecules like leukocyte function-associated antigen-1 (28). The NK cells usually form part of the body’s elimination response to tumours in the immunoediting hypothesis, and inhibition may result in escape of the immune response and uncontrolled tumour proliferation. Studies have shown increased numbers of the natural killer (NK) cells vital to immunomodulation and tumour suppression in rats inoculated with breast cancer cells and treated with propofol (28).
Despite the theories above, there has been little evidence from retrospective cohort trials regarding long term benefit of TIVA over inhalational anaesthesia, and results of cohorted randomised control trials are awaited. A large retrospective analysis in 2016 looked at mortality outcomes for patients who had received either TIVA or inhalational anaesthesia for all cancer surgery in patients over 18 years, with approximately 7,000 total patients over a 3-year period (29). However, there were notable differences in baseline characteristics of the two groups. Patients in the inhalational group were more likely to be male, have an ASA score of III or IV and have metastases. After propensity matching and adjustment for confounding factors, patients had worse outcomes following inhalational anaesthesia with an adjusted hazard ratio of 1.46 for death. Findings from this study were replicated in a retrospective analysis in Sweden, although findings were not statistically significant after adjustment for confounders (29). The limited available observational evidence suggests that TIVA has plausible potential benefits for improving outcomes after cancer surgery, however we await results of randomised-control trials to confirm or refute these findings.
Regional anaesthesia
Regional techniques are well understood to reduce the surgical stress response through inhibition of afferent inputs from surgical site to the central nervous system and hypothalamic-pituitary axis (2). Reduction of the stress response will moderate the immunosuppression and decrease in NK cells associated with increased risk of metastasis; studies in rats have shown that higher levels of NK cells correlate with reduced lung metastases following laparotomy (30). The presence of local anaesthetic solutions themselves may also be protective against metastasis. In vitro studies have shown that some local anaesthetics can damage cells’ cytoskeletal structure, which can affect tumour cells’ adhesive properties and thus their potential for metastatic spread (31). In addition to the anti-proliferative effects, some local anaesthetic drugs such as procaine and lidocaine also demethylate DNA in some breast cancer cell lines in vitro (32) and thus may contribute to reduction in tumour growth this way also. However, these in vitro findings are not reflected in recent in vivo studies. A recent study of paravertebral blocks and outcomes after breast cancer failed to demonstrate any benefit in risks of cancer recurrence. This was a large randomised-control trial over a ten -year period. Patients were randomised to receive a general anaesthetic with sevoflurane or paravertebral block with intravenous propofol. There was no difference in cancer recurrence rates at a median follow-up of 36 months (33).
Drug therapy (Table 2)
Table 2
| Drug | Implications |
|---|---|
| Opiates | Immuno-modulatory properties; chronic use of morphine in particular associated with decreased NK cells. |
| May increase likelihood of metastasis although lack of larger trials and no clear evidence | |
| Non-steroidal anti-inflammatories | Prostaglandins may increase cancer growth through increased interleukins, reduced apoptosis and angiogenesis |
| NSAIDs may have a role in decreasing prostaglandins, particularly COX-2 antagonists | |
| Anti-fibrinolytics | May reduce matrix metalloproteinases and thus metastasis |
| Beta-blockers | Inconsistent results, but potential role in reducing Beta mediated stress response in certain cancers e.g. melanoma |
Opioids
There have been several retrospective studies in this area suggesting that high opiate use in cancer surgeries is associated with poorer prognosis (34). Opiates have immunomodulatory properties, and chronic administration of morphine in particular is associated with decreased NK cells and cytokine expression (35). Some cancer cells (particularly breast, colon and lung) express µ-opioid receptors (MOR), a main target receptor for opiates. MOR agonism by morphine in vivo promotes the release of vascular endothelial growth factor (VEGF) and subsequently tumour growth volume and vascularisation (36). In vitro studies have shown that suppression of MOR with the MOR antagonist methylnaltrexone results in reduced tumour growth and metastasis (37).
Despite the above, there is conflicting evidence that morphine may have breast and colon tumour inhibiting properties via its effects on the regulation of matrix metalloproteinases (MMPs), enzymes that are involved in cell invasion and metastasis (38). Despite this, opiates remain the most commonly used perioperative analgesic (39), however many anaesthetic and surgical techniques use multi-modal approaches to analgesia, with a focus on opioid-sparing analgesia.
However, as with regional anaesthesia, there are a lack of large randomised control trials that show a clear risk or benefit to use of opiate analgesia. Indeed, retrospective trials looking at potential associations between opiate use and cancer recurrence have had mixed results with no clear evidence of a link sufficient to result in a change in current practice (1).
Non-steroidal anti-inflammatories
Prostaglandins and thromboxane A2 are key players in the body’s inflammatory response. They are derived from arachidonic acid by cyclo-oxygenase (COX) enzymes and production increases very rapidly in response to acute inflammation. COX-1 is found in most cells and has regulatory functions, but COX-2 is induced by inflammation, growth factors and hormones, and is the important source of prostaglandins in acute inflammation and cancers (40). Prostaglandins exert their effects via G protein receptors and have a vital role in regulation of the immune response. They are believed to promote cancer growth through their immunosuppressive effects, increased levels of inflammatory cytokines like interleukins 6 and 10, reduction of apoptosis and angiogenesis (1).
Non-steroidal anti-inflammatory drugs decrease localised and systemic prostaglandins through inhibition of the COX enzymes. There have been several studies looking at the effect of reducing prostaglandin production using in vitro and in vivo models (40). In vitro mouse models treated with the selective COX-2 inhibitor celecoxib have shown a reduction in angiogenesis and increase in apoptosis for breast cancer (41).
However, these promising models have not necessarily yielded conclusive results in human studies (42). A 3-year cohort study of over 2,000 patients receiving perioperative NSAIDs showed reduced risk of colorectal cancer recurrence with ibuprofen use but findings for diclofenac were not statistically significant (perhaps due to low numbers within the study receiving this drug) (43). NSAIDs are also associated with adverse effects and a further Danish cohort trial showed statistically significant increased rate of anastomotic leaks associated with perioperative use of diclofenac (44). There is observational evidence that peri-operative NSAIDs may improve disease free survival in breast cancer (45). However concerns over side-effects remain and a Cochrane review is underway looking at incidence of postoperative pain and haematoma formation with NSAID use in breast cancer patients, with cancer recurrence as a secondary outcome measure (46).
Despite a lack of conclusive evidence there does remain a strong role for the use of non-steroidal anti-inflammatory drugs as part of a multimodal analgesic programme on an individual basis.
β-Blockers
In theory, limiting β-adrenergic receptor activation during surgery should lessen the inflammatory effects caused by β cell mediated stress response and adrenergic axis. Several tumour lines have been shown to have over proliferation of β adrenergic receptors in vitro (42), and activation of these can have wide effects on the micro-environment of the tumour and lead to accelerated cancer spread in mouse models. Sympathectomy in animal models shows reduction in tumour growth of prostate cells and particularly tumour lymphatics (47). However, in vivo studies have shown inconsistent benefits of perioperative β blocker use and two separate large meta-analyses in 2018 suggested no link between perioperative β blocker use and generalised improved outcomes, although cancer-specific outcomes were more promising, and these drugs may have a role in treatment of cancers such as melanoma, ovarian cancer and pancreatic cancer (48). Many of the studies within the meta-analyses that have been published are retrospective, and there remains a role for prospective randomised control trials looking at β-blocker use in cancer recurrence, indeed several such studies are currently underway (49).
Antifibrinolytics
Some invasive cancers such as mesothelioma, oesophageal and pancreatic cancers express urokinase-type plasminogen activator (uPA). This has a role in converting serum plasminogen to plasmin, a protease that has activity within the seeding of metastatic cancer cells, particularly across the blood brain barrier (50). Anti-fibrinolytics like tranexamic acid and e-aminocaproic acid are synthetic lysine derivatives and inhibit the activation of plasmin. They also have a role in the reduction of matrix metalloproteinases (MMPs), the enzymes involved in cancer cell growth and invasion, as shown in breast cancer models in vitro (51). Use of these drugs regularly may have other benefits with respect to coagulation and prevention of excessive bleeding leading to increased requirement of blood product transfusion.
Blood transfusion
Allogenic blood transfusion introduces foreign antigens into the patient’s body which can have immunosuppressive and pro-inflammatory effects on the donor; these have collectively been described as transfusion related immunomodulation (TRIM) (52). TRIM includes release of prostaglandins, inhibition of interleukins like IL-2, and suppression of immune cells like cytotoxic cells and monocytes (53). In vitro models have shown that storage of red blood cells increases both the immunomodulatory response and tumour growth, whereas fresh red cells have no such effect, suggesting that hypoxia may compound this response.
In 2009, a large meta-analysis found a correlation between recurrence of colon cancer and allogenic blood transfusion perioperatively (54). However, although many studies in the area of blood transfusion generally show worse outcomes for cancer patients, this has not been conclusively linked to blood transfusion and TRIM and may be due to other factors such as perioperative anaemia, blood loss and surgical complications, which may in themselves be risk factors for poorer outcomes (1).
Conclusions
Despite the lack of definitive trial outcomes, there is plausible evidence that perioperative strategies to reduce the stress response during and after cancer surgery can potentially have wide reaching effects on cancer outcomes for patients. “Cancer” is an umbrella term that encompasses many different histological cell types making generalisation of evidence very difficult to interpret. It may be too early to develop robust evidence-based guidelines based upon the concepts within this article, however anaesthetists should be aware of how their perioperative practice can have wider implications for cancer recurrence in their patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Chris Jones and Leigh Kelliher) for the series “Perioperative Care of the Cancer Patient” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/dmr-20-94
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-20-94). The series “Perioperative Care of the Cancer Patient” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gottschalk A, Sharma S, Ford J, et al. The Role of the Perioperative Period in Recurrence After Cancer Surgery. Anesth Analg 2010;110:1636-43. [Crossref] [PubMed]
- Desborough JP.. The stress response to trauma and surgery Br J Anaesth 2000;85:109-17. [Crossref] [PubMed]
- Burton D, Nicholson G, Hall G. Endocrine and metabolic response to surgery Continuing Education in Anaesthesia Critical Care & Pain 2004;4:144-7. [Crossref]
- Finnerty CC, Mabvuure NT, Ali A, et al. The surgically induced stress response. JPEN J Parenter Enteral Nutr 2013;37:21S-9S. [Crossref] [PubMed]
- Gore DC, Jahoor F, Wolfe RR, et al. Acute response of human muscle protein to catabolic hormones. Ann Surg 1993;218:679-84. [Crossref] [PubMed]
- Ogawa K, Hirai M, Katsube T, et al. Suppression of Cellular Immunity by Surgical Stress. Surgery 2000;127:329-36. [Crossref] [PubMed]
- Morgan E. Regulation of Drug-Metabolizing Enzymes and Drug Metabolism by Inflammatory Responses. Drug Metabolism in Disease 2017;21-58.
- Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 2010;70:5649-69. [Crossref] [PubMed]
- Poste G, Fidler I. The pathogenesis of cancer metastasis. Nature 1980;283:139-46. [Crossref] [PubMed]
- Yamaguchi K, Takagi Y, Aoki S, et al. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg 2000;232:58-65. [Crossref] [PubMed]
- Armaiz-Pena GN, Lutgendorf S, Cole S, et al. Neuroendocrine modulation of cancer progression. Brain Behav Immun 2009;23:10-5. [Crossref] [PubMed]
- Armaiz-Pena GN, Cole S, Lutgendorf S, et al. Neuroendocrine influences on cancer progression. Brain Behav Immun 2013;30:S19-25. [Crossref] [PubMed]
- Yang EV, Sood AK, Chen M, et al. Norepinephrine Up-regulates the Expression of Vascular Endothelial Growth Factor, Matrix Metalloproteinase (MMP)-2, and MMP-9 in Nasopharyngeal Carcinoma Tumor Cells. Cancer Res 2006;66:10357-64. [Crossref] [PubMed]
- Langley RR, Fidler IJ.. Tumor Cell-Organ Microenvironment Interactions in the Pathogenesis of Cancer Metastasis. Endocr Rev 2007;28:297-321. [Crossref] [PubMed]
- Hiller J, Brodner G, Gottschalk A. Understanding clinical strategies that may impact tumour growth and metastatic spread at the time of cancer surgery. Best Pract Res Clin Anaesthesiol 2013;27:427-39. [Crossref] [PubMed]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565-70. [Crossref] [PubMed]
- Chan AS, Ng LW, Poon LS, et al. Dopaminergic and adrenergic toxicities on SK-N-MC human neuroblastoma cells are mediated through G protein signaling and oxidative stress. Apoptosis 2007;12:167-79. [Crossref] [PubMed]
- Sastry KS, Karpova Y, Prokopovich S, et al. Epinephrine Protects Cancer Cells from Apoptosis via Activation of cAMP-dependent Protein Kinase and BAD Phosphorylation. J Biol Chem 2007;282:14094-100. [Crossref] [PubMed]
- Hijazi Y, Gondal U, Aziz O. A systematic review of prehabilitation programs in abdominal cancer surgery. Int J Surg 2017;39:156-62. [Crossref] [PubMed]
- Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of Physical Activity on Cancer Recurrence and Survival in Patients With Stage III Colon Cancer: Findings From CALGB 89803. J Clin Oncol 2006;24:3535-41. [Crossref] [PubMed]
- Idorn M, Hojman P. Exercise-Dependent Regulation of NK Cells in Cancer Protection. Trends Mol Med 2016;22:565-77. [Crossref] [PubMed]
- Hojman P. Exercise protects from cancer through regulation of immune function and inflammation. Biochem Soc Trans 2017;45:905-911. [Crossref] [PubMed]
- Lidder PG, Sanders G, Whitehead E, et al. Pre-Operative Oral Iron Supplementation Reduces Blood Transfusion in Colorectal Surgery - A Prospective, Randomised, Controlled Trial. Ann R Coll Surg Engl 2007;89:418-21. [Crossref] [PubMed]
- Horowitz M, Neeman E, Sharon E, et al. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol 2015;12:213-26. [Crossref] [PubMed]
- Shi QY, Zhang SJ, Liu L, et al. Sevoflurane promotes the expansion of glioma stem cells through activation of hypoxia-inducible factors in vitro. Br J Anaesth 2015;114:825-30. [Crossref] [PubMed]
- Evans MT, Wigmore T, Kelliher LJS. The impact of anaesthetic technique upon outcome in oncological surgery. BJA Educ 2019;19:14-20. [Crossref]
- Yan T, Zhang GH, Wang BN, et al. Effects of propofol/remifentanil-based total intravenous anesthesia versus sevoflurane-based inhalational anesthesia on the release of VEGF-C and TGF-β and prognosis after breast cancer surgery: a prospective, randomized and controlled study. BMC anesthesiol 2018;18:131. [Crossref] [PubMed]
- Tazawa K, Koutsogiannaki S, Chamberlain M, et al. The effect of different anesthetics on tumor cytotoxicity by natural killer cells. Toxicol Lett 2017;266:23-31. [Crossref] [PubMed]
- Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile vs. IV anaesthesia for cancer surgery: A retrospective analysis. Anesthesiology 2016;124:69-79. [Crossref] [PubMed]
- Melamed R, Rosenne E, Shakhar K, et al. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a β-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun 2005;19:114-26. [Crossref] [PubMed]
- Xuan W, Hankin J, Zhao H, et al. The potential benefits of the use of regional anesthesia in cancer patients. Int J Cancer 2015;137:2774-84. [Crossref] [PubMed]
- Lirk P, Berger R, Hollmann MW, et al. Lidocaine time- and dose-dependently demethylates deoxyribonucleic acid in breast cancer cell lines in vitro. Br J Anaesth 2012;109:200-7. [Crossref] [PubMed]
- Sessler DI, Pei L, Huang Y, et al. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet 2019;394:1807-15. [Crossref] [PubMed]
- Connolly C, Buggy DJ. Opioids and tumour metastasis: does the choice of the anesthetic-analgesic technique influence outcome after cancer surgery? Curr Opin Anaesthesiol 2016;29:468-74. [Crossref] [PubMed]
- Grandhi RK, Lee S, Abd-Elsayed A. Does Opioid Use Cause Angiogenesis and Metastasis? Pain Med 2017;18:140-51. [Crossref] [PubMed]
- Gupta K, Kshirsagar S, Chang L, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res 2002;62:4491-8. [PubMed]
- Mathew B, Lennon FE, Siegler J, et al. The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg 2011;112:558-67. [Crossref] [PubMed]
- Sherwin A, Buggy DJ. The Effect of Anaesthetic and Analgesic Technique on Oncological Outcomes. Curr Anesthesiol Rep 2018;8:411-25. [Crossref]
- Cata J, Bugada D, Marchesini M, et al. Opioids and Cancer Recurrence. Cancer Cell and Microenvironment 2016;3:1-8.
- Menter DG, Schilsky RL, DuBois RN. Cyclooxygenase-2 and Cancer Treatment: Understanding the Risk Should Be Worth the Reward. Clin Cancer Res 2010;16:1384-90. [Crossref] [PubMed]
- Yoshinaka R, Shibata MA, Morimoto J, et al. COX-2 Inhibitor Celecoxib Suppresses Tumor Growth and Lung Metastasis of a Murine Mammary Cancer. Anticancer Res 2006;26:4245-54. [PubMed]
- Hiller JG, Parat MO, Ben-Eliyahu S. The Role of Perioperative Pharmacological Adjuncts in Cancer Outcomes: Beta-Adrenergic Receptor Antagonists, NSAIDs and Anti-fibrinolytics. Curr Anesthesiol Rep 2015;5:291-304. [Crossref]
- Schack A, Fransgaard T, Klein MF, et al. Perioperative Use of Nonsteroidal Anti-inflammatory Drugs Decreases the Risk of Recurrence of Cancer After Colorectal Resection: A Cohort Study Based on Prospective Data. Ann Surg Oncol 2019;26:3826-37. [Crossref] [PubMed]
- Klein M, Gögenur I, Rosenberg J. Postoperative use of non-steroidal anti-inflammatory drugs in patients with anastomotic leakage requiring reoperation after colorectal resection: cohort study based on prospective data. BMJ 2012;345:e6166. [Crossref] [PubMed]
- Forget P, Bentin C, Machiels JP, et al. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. Br J Anaesth 2014;113:i82. [Crossref] [PubMed]
- Klifto KM, Major MR, Leto Barone AA, et al. Perioperative systemic nonsteroidal anti‐inflammatory drugs (NSAIDs) in women undergoing breast surgery. Cochrane Database Syst Rev 2019;2019:CD013290. [Crossref]
- Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science 2013;341:1236361. [Crossref] [PubMed]
- Na Z, Qiao X, Hao X, et al. The effects of beta-blocker use on cancer prognosis: a meta-analysis based on 319,006 patients. Onco Targets Ther 2018;11:4913-44. [Crossref] [PubMed]
- Yap A, Lopez-Olivo MA, Dubowitz J, et al. Effect of beta-blockers on cancer recurrence and survival: a meta-analysis of epidemiological and perioperative studies. Br J Anaesth 2018;121:45-57. [Crossref] [PubMed]
- Perides G, Zhuge Y, Lin T, et al. The fibrinolytic system facilitates tumor cell migration across the blood-brain barrier in experimental melanoma brain metastasis. BMC Cancer 2006;6:56. [Crossref] [PubMed]
- Afsharimani B, Cabot PJ, Parat MO. Effect of lysine antifibrinolytics and cyclooxygenase inhibitors on the proteolytic profile of breast cancer cells interacting with macrophages or endothelial cells. Br J Anaesth 2014;113:i22-31. [Crossref] [PubMed]
- Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): An update. Blood Rev 2007;21:327-48. [Crossref] [PubMed]
- Cata JP, Wang H, Gottumukkala V, et al. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth 2013;110:690-701. [Crossref] [PubMed]
- Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev 2006;2006:CD005033. [Crossref] [PubMed]
Cite this article as: O’Rourke K, Huddart S. Surgical stress response and cancer outcomes: a narrative review. Dig Med Res 2020;3:65.