
Controlled expression of XRCC2 associated with induced DNA damage: a new hope in colorectal cancer
Colorectal cancer (CRC) is considered as the world’s fourth most deadly cancer with an estimated death of 900,000 annually. Besides ageing and lifestyle, dietary habit also contributes to the onset and the progress of this disease. Several treatment options are available which may be divided into a few major categories such as targeted drug therapy, surgical procedure, radiotherapy, chemotherapy and recently developed immunotherapy. However, all treatment procedures found to be less effective in patients with the metastasis-a process of developing secondary tumours in other organs apart for the primary site. Since the symptoms appear at the advanced stage of this disease; therefore, it is recommended to screen for the early detection to control the disease and subsequently to reduce the mortalities. Globally, it is accepted that the existing therapies are not enough to save this significant loss of lives and we are in the dire need to develop new targeted therapies.
Developing a targeted therapy needs in-depth research of identification and characterization of a potential target which plays an important role in the initiation or in the progress of the disease and may have relevance to the prognosis or diagnosis of the disease. In CRC, drugs were developed by targeting proteins such as growth factors (vascular endothelial growth factor or VEGF, epidermal growth factor receptor, EGFR), cellular enzymes such as protein kinases (phosphorylate proteins). However, most of the drugs have moderate to severe side effects such as diarrhoea, high blood pressure, weight loss, abdominal pain severe bleeding or perforation in the stomach or intestine. Therefore, it is needless to mention that new drug development is essential with much modest side effect and improved efficacy.
Now, one of the burning questions in the field of development of targeted therapies is that what are the potential areas that we should focus to find a novel target? Cancer is a genetically diverse disease and even these diversities are not very well documented in any types of cancer till today. It is widely accepted that the DNA damage checkpoint control mechanism is defective in most of the cancers and therefore, has emerged as a potential area of drug development to control cancer cell population (1,2). Mammalian cells possess an inbuilt mechanism to repair the breakage of single or double-stranded DNA which happens when cells are exposed to high energy radiation or chemical carcinogens. Once cell detects the damage then it activates one of the two kinases ATM (ataxia-telangiectasia, mutated) and ATR (ATM and Rad3-related) depending upon the types of damage. The activated ATM or ATR phosphorylates p53, a tumour suppressor protein which plays a major role in cell cycle regulation. The phosphorylated p53, the activated form of this protein, acts as a transcription factor and initiates transcription of several of its downstream genes such as p21, an inhibitor CyclinE-CDK2 and Puma, an inhibitor of anti-apoptotic protein Bcl2. Inhibition of CyclinE-CDK2 blocks the activation of E2F, the master regulator of transcription of the genes that control cell cycle at the G/S transition. Simultaneously, inhibition of Bcl2 activates the apoptosis, a process of irreversible cell death. The activation of p53 also initiates the repair pathways which are subdivided as base excision repair (BER), nucleotide excision repair (NER), mismatch repair (MMR), homologous recombination (HR) and non-homologous end-joining (NHEJ) and these pathways are activated throughout different stages of the cell cycle, allowing the cells to repair the damaged DNA. Each repair pathway is highly regulated and operated by a group of protein factors or co-factors.
So, the question remains, how the DNA damage checkpoint control pathway is considered as a potential target in cancer drug discovery? The genetic aberrations that predispose to cancer introduce mutations in the genes that are involved in the DNA repair. Proteins that are involved in this pathway become nonfunctional to respond to the signaling and the repair process and eventually trigger apoptosis. Therefore, one of the strategies to prevent cancer cell proliferation and triggering apoptosis is to expose cells to the high energy radiation or to DNA cross-linking agents such as Oxaliplatin to induce double-stranded DNA breakage. However, this is not a risk-free process because healthy cells surrounding the tumour may accumulate irrecoverable damage and could induce cell mortality. The cytotoxic drug, such as Oxaliplatin alone, creates a major side effect damaging normal tissues. It is very challenging, therefore, to treat solid tumours either with controlled radiation or with a cytotoxic drug such as Oxaliplatin without anticipating the side effects. One of the strategies to minimise the side effect of drugs is to introduce a combination of drug treatments. It is expected to produce less side effect because the dose of any single drug used in combination will be much less than its use as a standalone drug.
Ren et al. (3) of the Seventh Affiliated Hospital of Sun Yat-sen University, Shenzhen, China recently attempted one of such double drug experiments on CRC. The study included the application of two chemotherapeutic drugs Olaparib and Oxaliplatin. Olaparib is an inhibitor of the enzyme Poly (ADP-ribose) polymerase 1 (PARP1) which binds to the DNA breaks and attracts the DNA repair proteins at the site of damage to facilitate the repair by homologous repair (HR) pathway. Oxaliplatin is the latest derivative drug belongs to the cisplatin group and found to be very effective in introducing DNA-intrastrand, DNA-inter strand and DNA-protein crosslinking. The irreversible nature of this binding blocks the repair and the DNA synthesis by minimizing the binding of DNA repair proteins at the site of damage and eventually inducing the G2/M arrest. As a result, the apoptotic process starts upon relocating the proapoptotic protein Bax to the mitochondria followed by the Caspase-3 activation and the release of Cytochrome-C to the cytosol (4,5).
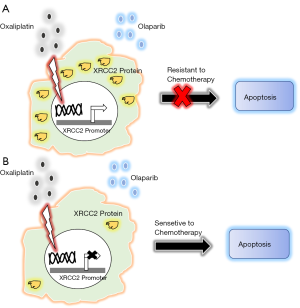
It is important to note that Ren et al. did not use both drugs on CRC cell because it was reported earlier that those cells are quite resistant to the chemotherapeutic drugs due to the expression XRCC2—a Rad51 family member protein and plays a critical role in the HR repair. This observation was further supported by the clinical data where patients with negative XRCC2 expression demonstrate more sensitivity to treatment with another chemotherapeutic drug such as 5-fluorouracil (5-FU) than those with positive XRCC2 expression (6). To avoid the interference of this protein, Ren et al. engineered an SW480 CRC cell with constitutive expression of shRNA targeting XRCC2 and used that cell line to perform a series of experiments to establish the effect of combined drugs. Collectively, Ren et al. study clearly demonstrated that the combination of drugs downregulated the ability to repair the DNA damage which was determined by the γ-H2AX foci formation in comparison to the single-drug treatments. In their subsequent experiments Ren et al. established that the double drug treatment is capable of also inhibiting the cell proliferation, induced G2/M arrest and enhanced apoptosis of this engineered SW480 CRC cells where the XRCC2 expression was reduced to almost 50% in comparison to the control.
This preliminary study of treating CRC cells with two chemotherapeutic drugs Olaparib and Oxaliplatin under the reduced XRCC2 expression condition perhaps would help us to develop a new therapeutic model in CRC. However, it is a long way to go before we find them to be clinically effective. It is evident from this and other relevant studies that the most critical component of this model would be to reduce the expression of XRCC2 or to functionally inactivate this protein at least partially to increase the sensitivity of chemotherapeutic drugs. XRCC2 is one of five somatic RAD51 paralogs and has a function in DNA double-strand break repair by HR. However, except RAD51, the function of XRCC2 as well other paralogs are not very well understood. Several studies in the lower eukaryotes indicated that XRCC2 forms a complex with other paralogs of Rad51 proteins and recruits the later on the DNA damage site. Though the shRNA mediated downregulation of gene expression is found to be very effective in cell line-based experiments but the success rate of using shRNA as a therapy is not very well established till today. Many studies are needed now to identify the partner proteins of XRCC2 participate in the complex formation and if possible, to identify small molecule inhibitors which could potentially block the XRCC2 for an active complex formation with its partner proteins to recruits Rad51 at the damage site. Another novel approach to reduce the synthesis of XRCC2 would be to target the regulatory mechanism that controls the transcription of XRCC2. In one of the recent studies by Chen et al. (7) demonstrated that the promoter of the XRCC2 is several hundred folds active in cancer cell than in normal cell and an effective downregulation of the promoter hyperactivity was successful to kill cancer cells. Analysis of XRCC2 promoters identified the binding site of the zinc finger transcription factor ZNF281 and characterized to be essential for the activation of the promoter (8). Therefore, it is possible that in cancer cells the promoter is occupied by several oncogenic protein factors or co-factors and which may occur due to certain changes in the conformation of the proximal and the distal part of the promoter. Such conditional binding of oncogenic factors or cofactors, which is limited in somatic cells, aggravate several folds of promoter activities. Identification and targeting one or more of such factors could be very effective to control the expression of this protein. According to this model, as shown in the figure, a new hope of treatment would be based on the combination treatment with chemotherapeutic drugs like Olaparib and Oxaliplatin under the reduced expression of endogenous XRCC2, to increase the lifespan of the patients with modest side effects (Figure 1).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Digestive Medicine Research. The article did not undergo external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-20-40). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Turgeon MO, Perry NJS, Poulogiannis G. DNA Damage, Repair, and Cancer Metabolism. Front Oncol 2018;8:15. [Crossref] [PubMed]
- Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature 2012;481:287-94. [Crossref] [PubMed]
- Ren H, Wu W, Li M, et al. Combined olaparib and oxaliplatin inhibits tumor proliferation by cell cycle arrest and cell apoptosis in XRCC2-defecient colorectal cancer. Dig Med Res 2019;2:41. [Crossref]
- Arango D, Wilson AJ, Shi Q, et al. Molecular mechanisms of action and prediction of response to oxaliplatin in colorectal cancer cells. Br J Cancer 2004;91:1931-46. [Crossref] [PubMed]
- Alcindor T, Beauger N. Oxaliplatin: a review in the era of molecularly targeted therapy. Curr Oncol 2011;18:18-25. [Crossref] [PubMed]
- Zhang YZ, An JH, Liu YX, et al. XRCC2-Deficient Cells are Highly Sensitive to 5-Fluorouracil in Colorectal Cancer. Cell Physiol Biochem 2017;43:1207-19. [Crossref] [PubMed]
- Chen Y, Li Z, Xu Z, et al. Use of the XRCC2 promoter for in vivo cancer diagnosis and therapy. Cell Death Dis 2018;9:420. [Crossref] [PubMed]
- Pieraccioli M, Nicolai S, Antonov A, et al. ZNF281 contributes to the DNA damage response by controlling the expression of XRCC2 and XRCC4. Oncogene 2016;35:2592-601. [Crossref] [PubMed]
Cite this article as: Mitra P. Controlled expression of XRCC2 associated with induced DNA damage: a new hope in colorectal cancer. Dig Med Res 2020;3:96.


