
Pancreatic metastasis of renal clear cell carcinoma: a case report and literature review
Overview
Pancreatic metastasis of renal clear cell carcinoma is relatively rare in clinic and has quite low incidence. Symptoms of this tumor have no specificity but strong concealment (1). Once diagnosed, almost all patients with the proposed tumor may have missed the best opportunity for treatment. In the case of a lack of specific examination method for diagnosis that results in missed best opportunity for diagnosis and treatment, it will cause irreversible results to the patients (2). The effective and timely diagnosis of pancreatic metastasis of renal clear cell carcinoma has a great influence on the effective follow-up treatment of patients. In addition, early treatment can help delay the death, prolong the survival time, and improve the survival rate of patients. At present, the major clinical diagnostic means is to judge the patient’s condition via imaging and relevant analysis based on imaging features (3). The characteristics of pancreatic metastasis of renal clear cell carcinoma correlate with the shape and type of the imaging results, but it still needs to be diagnosed by biopsy. In this paper, a retrospective analysis was carried out associated with the review of relevant literature to analyze the clinical characteristics of a case with pancreatic metastasis of renal clear cell carcinoma in our hospital recently, The patient underwent left nephrectomy for clear cell carcinoma of the left kidney 19 years ago, and metastatic carcinoma of the head of the pancreas was not found until 19 years after surgery.so as to improve the recognition of diagnosis for pancreatic metastasis of renal clear cell carcinoma and assist its treatment accordingly. We present the following case in accordance with the CARE-Guideline (4).
Data
The patient was a 65-year-old male who was admitted to our hospital due to the discovery of pancreatic mass during a physical examination. Before the physical examination found the tumor, the patient has no abdominal pain, abdominal distension and other symptoms, bilirubin, amylase is normal. No palpable masses were found on abdominal physical examination. The patient had a medical history of left nephrectomy for left renal clear cell carcinoma 19 years ago, and had underwent cholecystectomy. Besides, the patient also had a medical history of hypertension for over 20 years with the maximal blood pressure of 160/100 mmHg, and the patient was treated with oral administration of Amlodipine Besylate Tablets with blood pressure controlled at 120/80 mmHg. In addition, the patient had a medical history of prostatic hyperplasia for 10 years and received oral drug therapy of Tamsulosin Hydrochloride. Family history was unremarkable.
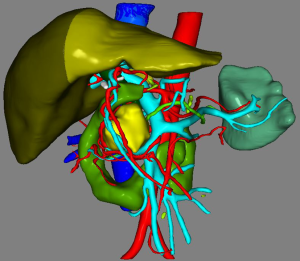
The patient received CT examination of the whole abdomen after admission, and the results suggested that: the head of pancreas showed significant localized enlargement with a slightly low-density mass locally (Figures 1,2), which was about 4.9×4.3×6.1 cm in size, showed clear boundary and sheet-like appearance. Besides, the arterial phase of enhanced scan showed significant enhancement, and the CT value was about 180–200 Hu. While the contrast medium was decreased in delayed period, but the enhanced intensity was still higher than that of the normal pancreatic tissue in the body and tail of pancreas, with the CT value of about 100 Hu. Additionally, there were some flaky and round-like weak enhancement areas within the focus in the arterial phase. The left gastric artery and pancreaticoduodenal artery around the focus were compressed and displace, accompanied by dilatation of pancreatic duct, common bile duct and left intrahepatic bile duct, considering the possibility of neuroendocrine tumor in pancreatic head mass. Furthermore, ultrasound guided puncture of pancreatic head tumor was performed in the patient, with pathological diagnostic finding of metastatic pancreatic renal clear cell carcinoma. Abdominal CT findings showed that the tumor was closely correlated with hepatic portal vein, showing the possibility of local wrapped invasion. The patient was considered to diagnosis of metastatic tumor of pancreatic head after multi-disciplinary discussion. but the mass is big and TNM stage belongs to IV, considering the close relationship with the hepatic portal vein, the tumor was not easy to be resected, and it was thus suggested to perform target therapy to reduce the tumor before surgery. Therefore, the patient was provided with 5 courses of conversion chemotherapy with apatinib and sunitinib. Patient develop granulocytosis and nausea during targeted therapy, after targeted therapy, the patient received CT for review, and the results when compared with those before targeted therapy suggested: the mass of the pancreatic head of 4.4×4.1×3.5 cm in size was mixed in nature, showed decreased density and had smaller volume than that before targeted therapy (Figures 3,4), showing local necrosis, slight expansion of pancreatic duct, common bile duct dilatation and intrahepatic bile duct dilatation of left hepatic duct and left lobe of liver, which were similar to those before targeted therapy. After concealing the pancreas by the 3D reconstruction of CT, it can be seen that the tumor is located in the head of the pancreas and closely related to the surrounding blood vessels (Figure 5). After complete preoperative preparation, the patient was provided with pancreatoduodenectomy. According to the intraoperative findings, the tumor was located in the head of pancreas, which was close to the hepatic portal vein. Postoperative resection of the specimen revealed that the tumor was located within the head of pancreas (Figure 6).
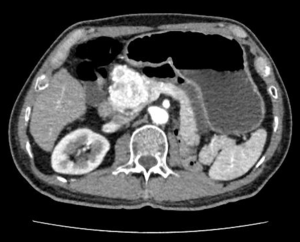
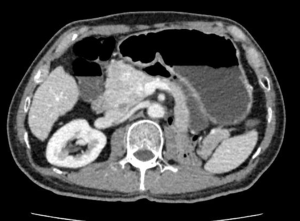
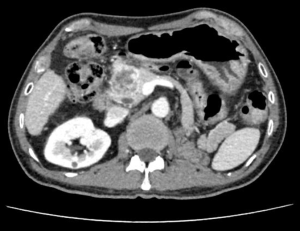
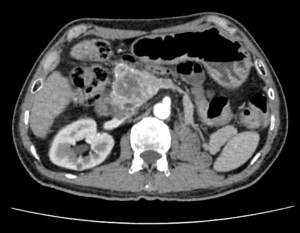
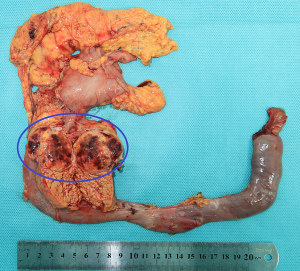
Postoperative pathological results: cancer cells were infiltrated in pancreatic tissue, with nest-like growth, rich cytoplasm, transparent appearance and rich interstitial blood vessels. Immunohistochemical results: 06E cancer cell CK (weak+), CK8/18 (weak+), CK7 (−), CK19 (−), CD10 (+), Vimentin (+), CgA (−), PAX-8 (+), PAX-2 (+), and RCC (−). Combined with medical history, morphology of HE and immunohistochemical results of the patient, the lesions were consistent with pancreatic metastasis of renal clear cell carcinoma. Meanwhile, no metastasis was found in the lymph node dissection of 20 lymph nodes.
Discussion
Approximately 50% of metastatic pancreatic tumors are induced by renal cell carcinoma, and 2–5% of which are metastatic tumors of the pancreas (5). The quantity of renal cell carcinoma spreading to pancreas is much lower than that to bone, lung, lymph node, liver, etc. (6). The symptoms of the tumor have no specificity but strong concealment. Most patients show no difference from ordinary population before the disease worsens. A small number of patients may have abdominal distention and pain, poor appetite, etc., which, however, are quite common in clinic. Patients often treat it as a common disease without much attention paid to, or even do not visit the hospital to consult a doctor and just get over-the-counter medication in the drugstore. In addition, there is an extremely few patients with obstruction of the common bile duct and pancreatic duct due to tumor invasion into the common bile duct and duodenum, resulting in obstructive jaundice and pancreatitis (7). Therefore, pancreatic metastasis of renal cell carcinoma is found during physical examination in most cases, and >50% of which occur only after several years of treatment for renal cell carcinoma. The incubation period of pancreatic metastasis of renal clear cell carcinoma is quite long, and it is reported that the longest period can reach 32.7 years after renal cell carcinoma resection. So far, it is still not clear with respect to the specific approach of renal cell carcinoma metastasizing to the pancreas, and it is speculated that there may be two ways of metastasis at present: (I) pancreas transfer via blood flow direction of blood vessel; and (II) invasion of the tumor into lymphatic vessels and metastasis to the pancreas. However, despite the above conjectures, there is not enough evidence to clarify the approach of pancreatic metastasis of renal clear cell carcinoma. In this regard, it is necessary to further strengthen the research in this field to prevent the spread of renal cell carcinoma to the pancreas from the source. At present, there is no specific index for predicting pancreatic metastasis of renal clear cell carcinoma in laboratory. This case of metastatic clear cell carcinoma of the head of the pancreas had a long incubation period, and the tumor was found in the physical examination before the onset of symptoms, and it was fortunate for the patient to have concurrent targeted therapy and surgical resection. Unfortunately, no pathway of tumor metastasis was found in this case.
Imaging examination may provide reference for the discovery of the disease. Lesions may be found in all parts of pancreas, either solitary or multiple. On the ultrasound image, the lesions were detected with hypoechoic mass mostly in pancreas, with clear, smooth and neat boundary, as well as regular shape (8). Furthermore, in color Doppler flow imaging, >4 punctate blood flow could be found in the mass, and only a few cases were located outside the outline of pancreas. Besides, on CT images, the masses were mostly round and oval in appearance (9). Over 50% of the masses showed low density on plain CT images, but significant enhancement in arterial phase. However, after entering the venous phase, the enhancement would be reduced, which was quite similar to that of pancreatic metastasis of renal clear cell carcinoma. In accordance with related literature, the image density of many renal clear cell carcinoma patients with pancreatic metastasis became stronger after entering the arterial phase, and there were some necrotic areas in the location of the disease. When patients were examined by MRI, necrosis might appear in this part of the area in the case of relatively lager tumor area. The type of this part was similar to that of ultrasound image. In addition, according to the results of MRI, the response of pancreatic metastasis of renal clear cell carcinoma was obviously weakened due to the presence of some fat in the tumor site. On the basis of the above image analysis, the characteristics of pancreatic metastasis of renal clear cell carcinoma also exhibited certain correlation with the shape and type of the image. However, there is no unified standard for clear diagnosis of pancreatic metastasis of renal clear cell carcinoma. The pathological information of pancreatic metastasis of renal clear cell carcinoma can not fully correspond to the information obtained by the diagnostic means, which deserves further explicit analysis.
After tumor resection, patients may develop directional metastasis within a few years., usually in pancreas with rich blood supply generally. In theory of ultrasound image, pancreatic metastasis of renal clear cell carcinoma is commonly compared with pancreatic neuroendocrine tumor. The majority of endocrine tumor images can be obtained from the ultrasound image, which are featured by obvious margin and larger volume in the mass when the patient’s condition progresses. Meanwhile, there is a high similarity in image signals of pancreatic neuroendocrine tumor and pancreatic metastasis of renal clear cell carcinoma commonly, it is therefore difficult to distinguish the proposed two types of diseases. However, some scholars have pointed out that there are many differences in the image information of the two diseases, with the presence of calcification in most cases within pancreatic neuroendocrine tumor. Meanwhile, there is great significance in magnetic resonance signals of the two disease. For instance, image signals of pancreatic metastasis of renal clear cell carcinoma are generally weakened in the contrary phase, but not in pancreatic neuroendocrine tumor.
At present, ultrasound puncture is considered to be the most commonly used diagnostic technique for pancreatic metastasis of renal clear cell carcinoma. However, there may be some diagnostic errors due to the limitation of the area tested by ultrasound puncture. At present, pathological examination after tumor resection has been accepted as the authoritative diagnostic approach. Further immunohistochemistry in tissues shall be carried out for areas that are not involved in pathological examination.
There is still a lack of unified authentication for the treatment of pancreatic metastasis of renal clear cell carcinoma. It has been found that there is little difference in the therapeutic effect between surgical resection and targeted therapy in patients with pancreatic metastasis of renal clear cell carcinoma. However, for the isolated metastatic tumor, the surgical treatment has a good effect. In this case, after 5 courses of targeted therapy, the tumor continued to grow during the one-month preparation for the surgery after drug withdrawal, so the appropriate time should be selected for the surgical treatment after targeted therapy. Besides, other scholars have proposed to establish a prediction model, which, however has not been verified and requires further exploration.
The whole treatment period lasted for half a year. Patients complained of decreased appetite, granulocytosis, decreased immunity and susceptibility to colds during targeted therapy. The patient was nervous and had insomnia the day before the operation. Postoperative abdominal pain was obvious, but postoperative recovery was smooth without complications.
To sum up, preoperative needle biopsy and postoperative pathological examination are accepted as the common approaches for the diagnosis of pancreatic metastasis of renal clear cell carcinoma, with resection as the most commonly used therapeutic method. Other treatment methods are also being explored, and targeted therapy can also be used as an alternative treatment. There is still a need for joint efforts of doctors and researchers to realize the diagnosis and research of pancreatic metastasis of renal clear cell carcinoma, so as to screen a more effective treatment plan.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr.2019.12.03). YH serves as the Editor-in-Chief of Digestive Medicine Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sperti C, Moletta L, Patanè G. Metastatic tumors to the pancreas: The role of surgery. World J Gastrointest Oncol 2014;6:381-92. [Crossref] [PubMed]
- Tosoian JJ. Resection of isolated renal cell carcinoma metastases of the pancreas: outcomes from the Johns Hopkins Hospital. J Gastrointest Surg 2014;18:542-8. [Crossref] [PubMed]
- Tang X, Liu S, Chen W, et al. A case of metastatic renal clear cell carcinoma of the pancreas with recurrent pancreatitis as the main manifestation. Chinese Journal of Hepatobiliary Surgery 2019;25:382-3.
- Riley DS, Barber MS, Kienle GS, et al. CARE 2013 Explanations and Elaborations: Reporting Guidelines for Case Reports. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Zhao J, Alimu P, He H, et al. Clinical diagnosis and treatment of adrenal metastatic tumor (report of 55 cases). Chinese Journal of Urology 2019;40:272-6.
- Xing R, Qin Z. Matrix of pancreatic ductal adenocarcinoma cells. Journal of International Oncology 2018;45:696-8.
- Zheng X, Qiu X, Shao Z, et al. Changes of serum CEMIP ca19-9 and CA242 in patients with pancreatic cancer and their clinical significance. Journal of Pracrical Hepatology 2019;22:280-4.
- Wang X, Cheng H, Li Y, et al. Experimental study on the preparation of rat whole pancreas acellular matrix stents by perfusion method. Chinese Journal of General Surgery 2016;31:1034-7.
- Yao X, Chen X, Xu J, et al. One case of renal clear cell carcinoma with gallbladder metastasis and literature review. Chinese Journal of Hepatobiliary Surgery 2019;25:127-8.
Cite this article as: Li J, Liu Y, Deng T, Yang S, Chen J, Wu W, He Y. Pancreatic metastasis of renal clear cell carcinoma: a case report and literature review. Dig Med Res 2019;2:40.


