
The role of the class I Wnt pathway antagonist sFRP4 in colorectal cancer
Colorectal cancer is a common malignancy in humans. The occurrence of colorectal cancer involves abnormal mutation of multiple genes. A large number of cellular signal transduction pathways are related to colorectal cancer. Among these pathways, mutations and epigenetic alterations in the Wnt/β-catenin, Hedgehog, transforming growth factor β (TGF-β)/Smads and phosphoinositide 3-kinase (PI3K)/Akt signaling pathways are important molecular factors in the occurrence of colorectal cancer. The Wnt signaling pathways play an important role in the development and progression of colorectal cancer, and the early development of 90% of colorectal cancers has been reported to be related to abnormal activation of the Wnt pathways (1,2). Secreted frizzled related protein 4 (sFRP4) is a class I antagonist of the Wnt signaling pathways and may serve as a genetic marker for early diagnosis and treatment of colorectal cancer.
Abnormal activation of the Wnt pathways leads to colorectal cancer development and progression
The Wnt pathways, which are important for regulation of cell growth, development and differentiation, form a highly complex protein interaction network. Abnormal activation of Wnt pathways is closely related to the occurrence of numerous tumors [such as colorectal, ovarian, lung, breast, cervical and prostate tumors (1,3,4)], especially colorectal cancer (1,2).
The Wnt pathways are classified into the canonical (Wnt/β-catenin) pathway and the noncanonical (Wnt/planar cell polarity or Wnt/Ca2+) pathway. All Wnt signaling are activated by the binding of a Wnt-protein ligand to a frizzled family receptor (Frz/Fz), co-receptor low-density lipoprotein (LDL) receptor related protein 5/6 (LRP5/6) are required for mediating the interaction between Wnt and Fz. Binding of the Wnt protein to the receptors allows transduction of signals to the second messenger protein dishevelled (Dsh). Activated Dsh protein inhibits the activity of the axis inhibition (Axin)/adenomatous polyposis coli (APC)/glycogen synthase kinase-3β (GSK-3β) complex composed of the scaffold protein Axin, APC protein and glycogen synthase kinase-3β (GSK-3β), preventing β-catenin from being degraded. A large amount of free β-catenin enters the nucleus through the nuclear membrane and acts on T cell factor (TCF)/lymphoid enhancing factor (LEF), which induces transcriptional activation, initiates expression of the downstream target genes C-myc and cyclin D1, and eventually results in malignant transformation of cells and tumorigenesis (5,6) (Figure 1).
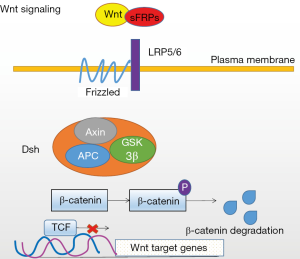
Mutations in the APC and β-catenin genes are widely present in colorectal cancer and lead to abnormal activation of the canonical Wnt signaling pathway and nuclear accumulation of β-catenin protein (6,7). To date, a variety of Wnt/β-catenin pathway-related target genes have been discovered. These target genes, such as the matrix metalloproteinase-7 (MMP-7), survivin, C-myc, cyclin D1, peroxisome proliferator activated receptor γ (PPAR-γ), cyclooxygenase-2 (COX-2), and vascular endothelial growth factor (VEGF) genes, are related to colon cancer cell apoptosis, growth, angiogenesis, invasion and metastasis (8,9). Blocking Wnt signaling pathway activity inhibits colon cancer cell proliferation (10). In addition, abnormal activation of the Wnt signaling pathways is related to the sensitivity of rectal cancer to radiochemotherapy (11). Thus, in-depth study of Wnt signaling pathways is conducive to the development of new methods for prevention and treatment of colorectal cancer.
The sFRP gene family antagonizes the Wnt pathway
Abnormal activation of Wnt pathways is often related to inactivation and downregulation of Wnt antagonists. The Wnt pathways are regulated extracellularly by secretory proteins classified as either class I or class II antagonists. Class I antagonists include Cerberus, sFRPs, and Wnt inhibitory factor 1 (WIF-1). Class I antagonists directly bind to Wnt, thereby antagonizing both the canonical and noncanonical Wnt pathways. Class II antagonists include the Dickkopf (DKK) family. DKKs inhibit Wnt signaling through binding to LRP5/6. Therefore, DKKs only antagonize the canonical Wnt pathway.
The sFRP gene family is the first discovered antagonist of the canonical Wnt pathway. sFRPs are secreted protein antagonists that bind directly to Wnt proteins.
sFRP1, sFRP2, and sFPR4, possess a conserved Frz-type cysteine-rich domain that binds Wnts and typically antagonize the Wnt signaling, presumably by preventing Wnt/Frz interactions. Thus SFRPs, except sFRP3, can function as antagonists of Wnt signalling by competing with Wnt proteins for binding to their receptor, Frz. These interactions prevent Wnt proteins from binding to Frz or lead to the formation of nonfunctional complexes with Frz, thereby blocking Wnt signaling. In colorectal cancer cell lines, sFRPs can attenuate Wnt signaling even in the presence of downstream gene mutations (12). The sFRP4 protein is composed of 349 amino acids and has a molecular weight of 39.9 kDa, which is the highest molecular weight among the members of the sFRP family. sFRP4 is expressed in the intestinal tract, endometrium, pancreas, stomach, liver, heart and mammary gland (13). Numerous studies have shown that sFRP4 regulates apoptosis and differentiation of tumor cells (14) and is an important antagonist of the Wnt pathway. However, there is a lack of systematic studies of sFRP4 in the literature.
The biological functions of sFRP4
SFRP4 is abnormally expressed in malignant tumors and differentially expressed in colorectal cancer
Loss of sFRP4 expression has been observed in most tumors, including ovarian cancer, malignant pituitary tumors, endometrial cancer, and acute myeloid leukemia (14). However, sFRP4 expression is upregulated in chemotherapy-sensitive ovarian cancer (15) and breast cancer (16). In addition, the expression of sFRP4 in these tumors is affected by varying degrees of methylation at its promoter region. Hypermethylation of the sFRP4 promoter occurs in ovarian cancer, pancreatic cancer, prostate cancer, renal cell carcinoma, and colorectal cancer (15).
The occurrence of colorectal cancer is an extremely complex process that involves polygenic changes. In addition to gene mutations, epigenetic alteration-induced silencing of tumor suppressor genes is also an important tumorigenic mechanism. A high degree of DNA methylation at the CPG islands of the promoter regions of tumor suppressor genes is a common feature of human tumors and may occur at various tumor stages. Hypermethylation of the sFRP family of genes, which are class I antagonists of the Wnt pathways, occurs in the early stages of colorectal tumors. SFRP4 contains abundant CPG islands in its exon regions. Therefore, gene silencing caused by DNA hypermethylation of sFRP4 may be an important reason behind the development and progression of colorectal tumors.
Colorectal cancer studies have yielded controversial results regarding the expression of sFRP4 in colorectal cancer. Our previous study found that sFRP4 expression was downregulated in 61.1% of colorectal cancer tissues and 9.1% of colorectal adenomas in comparison with normal tissues. In colon cancer, the incidence of DNA methylation of the sFRP4 gene was found to be 36.1% (26/72), which was significantly different from that in normal mucosa (17). In contrast, Huang et al. employed fluorescence-based quantitative polymerase chain reaction (PCR), immunoblotting and immunohistochemical techniques to systematically investigate the expression of sFRPs in large intestinal carcinoma (18). The results showed that the immunostaining level of sFRP4 was rather low in the cytoplasm of normal mucosal cells and adenoma cells, while sFRP4 was highly expressed in high-grade intraepithelial neoplasms and large intestinal carcinoma tissues. sFRP4 was overexpressed in 80% of the patients with large intestinal carcinoma and 42% of the patients with adenomas. These two contradictory results may be explained by pathological differences among the collected colorectal cancer specimens, the number of colorectal cancer specimens and the choice of experimental methods.
In addition, the expression of sFRP1, sFRP2 and sFRP5 has been reported to be elevated in SW480 colorectal cancer cells after downregulation of β-catenin expression, while the expression of sFRP4 was not significantly altered (13). However, one study showed that treatment to induce DNA demethylation of sFRP4 resulted in inhibition of Wnt signaling pathways (15). Thus, controversies still exist surrounding the role of sFRP4 in the early stage of colorectal cancer development and the factors correlated with sFRP4 expression levels. Epigenetics is a reversible process. Defining the roles of sFRP4 DNA methylation and gene expression in colorectal cancer may provide a new breakthrough point for treatment of colorectal cancer.
sFRP4 is a potential target for tumor gene therapy
sFRP4 expression may be correlated with the prognosis of tumor patients. Prostate cancer patients with high sFRP4 mRNA content show a better prognosis and a lower recurrence rate (19). In addition, sFRP4 may promote apoptosis and differentiation of tumor cells (15). sFRP4 expression is also related to the degree of chemosensitivity. Warrier et al. reported that the sFRP4 gene induced apoptosis of cancer stem cells (CSCs) in head and neck squamous cell carcinoma (20) and reduced the expression of the stem cell markers cluster of differentiation 44 (CD44) and aldehyde dehydrogenase (ALDH), thereby inhibiting the pluripotent differentiation of CSCs, increasing the sensitivity of tumors to chemotherapeutic drugs, and reducing drug resistance. In glioblastoma multiforme, introduction of the sFRP4 gene in combination with anti-tumor drugs increased the expression of the apoptotic markers Bcl-2-associated X (BAX) and p21, downregulated the expression of the differentiation-promoting protein cyclin D1, and effectively inhibited the activity of the Wnt pathway (21). Introduction of the sFRP4 gene in endometrial cancer effectively inhibited activation of the Wnt pathways through regulation of Wnt7a protein (22). Saran et al. proposed that sFRP4 is an important gene for evaluating the sensitivity of ovarian cancer to chemotherapeutic drugs and that sFRP4 expression was directly proportional to the degree of chemotherapeutic drug sensitivity (23,24). Therefore, upregulation of sFRP4 expression might be conducive to treatment of chemotherapeutic drug-resistant ovarian cancers.
On the other hand, sFRP4 may be a key molecule at the intersection of the Wnt pathways and other pathways and be closely related to tumor formation, invasion and infiltration. The expression of sFRP4 is negatively correlated with expression of the P53 tumor suppressor gene. Loss of sFRP4 expression induces silencing of the COX2 gene and the mismatch repair gene MLH1 and upregulates the expression of MMP2 and MMP9, which are capable of promoting tumor cell invasion and infiltration; thus, loss of sFRP4 expression promotes tumor cell invasion and infiltration. In addition, sFRP4 activates the anti-apoptotic Akt/protein kinase B (PKB) signaling pathway (25,26).
The role of blocking sFRP4 expression in treatment of colorectal tumors
Conventional chemotherapeutic drugs for colorectal cancer include oxaliplatin and 5-fluorouracil (5-FU). Most chemotherapeutic drugs cause serious side effects, such as decreased white blood cell count, loss of appetite, severe hair loss, and liver/kidney toxicity. In recent years, as molecular biology has advanced, researchers have gradually focused on gene therapy. Blocking of the Wnt pathways is a very important step in treatment of colorectal cancer, and for this purpose, the genes in the sFRP family are considered potential targets. A study conducted by Chen et al. showed that downregulation of sFRP4 expression decreased the proliferation rate of colorectal cancer cells (27). Miao et al. proposed that methyl CpG binding protein 2 (MeCP2) effectively regulates the methylation level of sFRP4 (28) and found that interference with MeCP2 expression increased sFRP4 expression, which led to a reduction in expression of the downstream protein β-catenin. As a result, activation of the Wnt pathway was blocked.
As a class I antagonist of the Wnt pathways, sFRP4 displays DNA hypermethylation in the early stages of colorectal cancer. sFRP4 is differentially expressed in colorectal cancer. However, the expression of sFRP4 is controversial in CRC. But blocking sFRP4 expression effectively reduces the proliferation rate of colorectal cancer cells. In addition, sFRP4 is related to chemoresistance in a variety of tumors and may serve as a potential prognostic marker. Epigenetics is reversible, and reversal of sFRP4 methylation is expected to antagonize Wnt pathway activation, thereby exerting a colorectal tumor-preventing effect.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr.2019.08.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Farooqi AA, de la Roche M, Djamgoz MBA, et al. Overview of the oncogenic signaling pathways in colorectal cancer: Mechanistic insights. Semin Cancer Biol 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Hankey W, Frankel WL, Groden J. Functions of the APC tumor suppressor protein dependent and independent of canonical WNT signaling: implications for therapeutic targeting. Cancer Metastasis Rev 2018;37:159-72. [Crossref] [PubMed]
- Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 2013;13:11-26. [Crossref] [PubMed]
- Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene 2017;36:1461-73. [Crossref] [PubMed]
- Nusse R, Clevers H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017;169:985-99. [Crossref] [PubMed]
- Steinhart Z, Angers S. Wnt signaling in development and tissue homeostasis. Development 2018;145:v146589. [Crossref] [PubMed]
- Cheng X, Xu X, Chen D, et al. Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed Pharmacother 2019;110:473-81. [Crossref] [PubMed]
- Rennoll SA, Konsavage WJ, Yochum GS. Nuclear AXIN2 represses MYC gene expression. Biochem Biophys Res Commun 2014;443:217-22. [Crossref] [PubMed]
- Gopalakrishnan N, Saravanakumar M, Madankumar P, et al. Colocalization of beta-catenin with Notch intracellular domain in colon cancer: a possible role of Notch1 signaling in activation of CyclinD1-mediated cell proliferation. Mol Cell Biochem 2014;396:281-93. [Crossref] [PubMed]
- Michels BE, Mosa MH, Grebbin BM, et al. Human colon organoids reveal distinct physiologic and oncogenic Wnt responses. J Exp Med 2019;216:704-20. [Crossref] [PubMed]
- Emons G, Spitzner M, Reineke S, et al. Chemoradiotherapy Resistance in Colorectal Cancer Cells is Mediated by Wnt/beta-catenin Signaling. Mol Cancer Res 2017;15:1481-90. [Crossref] [PubMed]
- Suzuki H, Watkins DN, Jair KW, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet 2004;36:417-22. [Crossref] [PubMed]
- Pawar NM, Rao P. Secreted frizzled related protein 4 (sFRP4) update: A brief review. Cell Signal 2018;45:63-70. [Crossref] [PubMed]
- Deshmukh A, Arfuso F, Newsholme P, et al. Epigenetic demethylation of sFRPs, with emphasis on sFRP4 activation, leading to Wnt signalling suppression and histone modifications in breast, prostate, and ovary cancer stem cells. Int J Biochem Cell Biol 2019;109:23-32. [Crossref] [PubMed]
- Pohl S, Scott R, Arfuso F, et al. Secreted frizzled-related protein 4 and its implications in cancer and apoptosis. Tumour Biol 2015;36:143-52. [Crossref] [PubMed]
- Karim RZ, Gerega SK, Yang YH, et al. Proteins from the Wnt pathway are involved in the pathogenesis and progression of mammary phyllodes tumours. J Clin Pathol 2009;62:1016-20. [Crossref] [PubMed]
- Qi J, Zhu YQ, Luo J, et al. Hypermethylation and regulation of expression of secreted frizzled-related protein genes in colorectal tumor. Zhonghua Zhong Liu Za Zhi 2007;29:842-5. [PubMed]
- Huang D, Yu B, Deng Y, et al. SFRP4 was overexpressed in colorectal carcinoma. J Cancer Res Clin Oncol 2010;136:395-401. [Crossref] [PubMed]
- Horvath LG, Henshall SM, Kench JG, et al. Membranous expression of secreted frizzled-related protein 4 predicts for good prognosis in localized prostate cancer and inhibits PC3 cellular proliferation in vitro. Clin Cancer Res 2004;10:615-25. [Crossref] [PubMed]
- Warrier S, Bhuvanalakshmi G, Arfuso F, et al. Cancer stem-like cells from head and neck cancers are chemosensitized by the Wnt antagonist, sFRP4, by inducing apoptosis, decreasing stemness, drug resistance and epithelial to mesenchymal transition. Cancer Gene Ther 2014;21:381-8. [Crossref] [PubMed]
- Schiefer L, Visweswaran M, Perumal V, et al. Epigenetic regulation of the secreted frizzled-related protein family in human glioblastoma multiforme. Cancer Gene Ther 2014;21:297-303. [Crossref] [PubMed]
- Carmon KS, Loose DS. Secreted frizzled-related protein 4 regulates two Wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol Cancer Res 2008;6:1017-28. [Crossref] [PubMed]
- Saran U, Arfuso F, Zeps N, et al. Secreted frizzled-related protein 4 expression is positively associated with responsiveness to cisplatin of ovarian cancer cell lines in vitro and with lower tumour grade in mucinous ovarian cancers. BMC Cell Biol 2012;13:25. [Crossref] [PubMed]
- Deshmukh A, Kumar S, Arfuso F, et al. Secreted Frizzled-related protein 4 (sFRP4) chemo-sensitizes cancer stem cells derived from human breast, prostate, and ovary tumor cell lines. Sci Rep 2017;7:2256. [Crossref] [PubMed]
- Constantinou T, Baumann F, Lacher MD, et al. SFRP-4 abrogates Wnt-3a-induced beta-catenin and Akt/PKB signalling and reverses a Wnt-3a-imposed inhibition of in vitro mammary differentiation. J Mol Signal 2008;3:10. [Crossref] [PubMed]
- Bhuvanalakshmi G, Gamit N, Patil M, et al. Stemness, Pluripotentiality, and Wnt Antagonism: sFRP4, a Wnt antagonist Mediates Pluripotency and Stemness in Glioblastoma. Cancers (Basel) 2018; [Crossref] [PubMed]
- Chen K, Liang H, Peng J, et al. Expression of secreted frizzled-related protein 4 in DNA mismatch repair-deficient and mismatch repair-proficient colorectal cancers. Nan Fang Yi Ke Da Xue Xue Bao 2018;38:1300-5. [PubMed]
- Miao C G, Huang C, Huang Y, et al. MeCP2 modulates the canonical Wnt pathway activation by targeting SFRP4 in rheumatoid arthritis fibroblast-like synoviocytes in rats. Cell Signal 2013;25:598-608. [Crossref] [PubMed]
Cite this article as: Liu Y, Li J, Qi J. The role of the class I Wnt pathway antagonist sFRP4 in colorectal cancer. Dig Med Res 2019;2:18.


