Post-operative bleeding complications in laparoscopic sleeve gastrectomy: sources, solutions and lessons learnt from a single cohort of patients
Introduction
Laparoscopic sleeve gastrectomy (LSG) was introduced in the early 1990s as part of biliopancreatic diversion with duodenal switch. In the early 2000s, it was used as a stand-alone procedure, with some concerns about its long-term results. The procedure has since gained popularity due to its benefits of preserving gastro-intestinal continuity, avoiding foreign body insertion, reduced risk of malabsorption, and offers a good option of conversion to multiple bariatric procedures. Outcomes are generally good with 50–80% excess weight loss (1,2).
Early post-operative complications after LSG that need to be identified include bleeding, staple line leak and development of an abscess. Delayed complications include strictures, nutritional deficiencies and gastroesophageal reflux disease (3). While there tends to be more focus on reduction of post-operative leak, there is paucity in the literature on the management of post-operative bleeding.
The sleeve gastrectomy encompasses the longest gastric staple line of any procedure and runs closest to the lesser curvature blood supply. As such, it is more pre-disposed to bleed intra- and post-operatively (4). Bleeding may arise from various sources (staple line, port sites, divided short gastric arteries, etc.) and poses a unique challenge in patient management. The prevention of bleeding with sleeve gastrectomy begins in the preoperative period and continues post-operatively.
The incidence of post-operative bleeding is reported up to 10%, highlighting that it is a significant problem to target (5). In this study, we aim to review bleeding complications in LSG and management of post-operative bleed including investigations, operative, non-operative and endovascular solutions.
Methods
A retrospective study of all LSG patients performed in a tertiary hospital, Singapore General Hospital (SGH) since 2008. Over 8 years, 409 patients who underwent sleeve gastrectomy performed by 4 surgeons were identified. These patients were placed on a low-calorie diet with Optifast 2 weeks prior to undergoing the surgery. Weight loss before surgery was recommended but not mandated. Post-operative bleeding is defined as requiring blood transfusion or re-laparoscopy to treat post-operative bleeding. Patients who encountered post-operative bleeding complications from LSG were identified from a prospectively recorded database. The presentation, source of bleeding and methods to stop the bleeding were recorded and presented.
Results
In the study period of 8 years from Dec 2008 to March 2016, 409 patients who underwent a sleeve gastrectomy by 4 surgeons were selected. This includes 151 males (37%) and 258 females (63%). The patient demographics, operation data and co-morbidities are elaborated in Table 1. Table 2 reflects other post-operative complications that were encountered, of which bleeding is the main topic of discussion.
Table 1
| Patient characteristics | n=409 |
|---|---|
| Age [range], years | 38 [18–66] |
| BMI [range], kg/m2 | 41.5 [30.8–82.1] |
| Sex ratio, female/male | 258/151 |
| Comorbidities, n (%) | |
| Hypertension | 170 (41.6) |
| Diabetes | 141 (34.5) |
| Dyslipidemia | 105 (25.7) |
| Obstructive sleep apnoea | 117 (28.6) |
| Fatty liver | 62 (15.2) |
Table 2
| Post-operative complications | n=409 |
|---|---|
| Bleeding, n (%) | 5 (1.2) |
| Sleeve migration, n (%) | 1 (0.2) |
| Abscess, n (%) | 1 (0.2) |
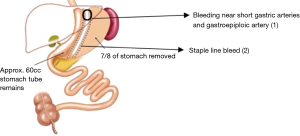
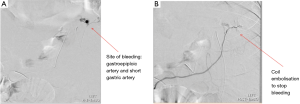
Of the 409 patients recruited for the study, 5 patients (1.2%) were diagnosed with post-operative bleeding, 4 of whom were males and 1 female. Two of these patients was treated conservatively requiring only blood transfusion and 3 patients were surgically explored with laparoscopy, no open conversions were required. The time to re-operation was 12–33 hours from index operation. Of these 3 patients, 2 of the bleeds were from staple line bleeding (SLB) and suture haemostasis performed. In 1 patient, the source of bleeding was not identified intraoperatively, and CT angiography performed post-operatively showed active contrast bleeding near short gastric arteries and gastroepiploic artery (Figure 1). Mesenteric angiogram with cannulation of the right gastroepiploic artery and short gastric artery via splenic artery showed active bleeding near the left diaphragm (Figure 2A). Gel foam and coil embolization was performed, and the bleeding was controlled (Figure 2B). For these patients that experienced post-operative bleeding, average hospitalization stay was 7.3 days (range, 6–9 days) as compared to 2.3 days (range, 1–7 days) for those without.
Discussion
The incidence of the bleeding following LSG is 0–10% (6). The stomach is richly supplied by a network of sub-mucous plexus, derived from the left and right gastric arteries, gastroepiploic vessels and short gastric arteries. Bleeding can occur following division of branches of the gastroepiploic and short gastric arteries. Alternatively, bleeding can also occur along the staple line. This can be attributed to inadequate compression haemostasis with staplers due to too large staple height compared with tissue thickness or vessels or patient factors such as abnormal clotting (4). Pre-operative patient optimization, intra-operative surgical methods and post-operative management play a role in reducing bleeding risk. Management of post-operative bleed will then be elaborated upon, including investigations, operative, non-operative and endovascular solutions.
Patients undergoing bariatric surgery often present with multiple comorbidities, hence, pre-operative optimisation of patient is crucial. This includes checking for coagulopathy, judicious use of venous thromboembolism prophylaxis and optimizing hydration status.
In addition, routine pre-operative ‘liver shrinking’ diet to reduce the size of the liver to make access to the stomach technically easier should be implemented (7). Studies have shown that even trivial weight loss over a short period of time can significantly reduce hepatic steatosis in obese patients and cross-sectional imaging studies have also demonstrated that visceral adipose tissue volume can be significantly reduced by short-term weight loss, allowing for easier elevation of the left liver lobe to expose the stomach and gastroesophageal junction, an essential step in the operation. Ultimately, the study conducted on overall complexity of bariatric surgery in patients with or without pre-operative weight loss to shrink their liver size revealed surgeons scoring lower overall complexity of surgery in the group with pre-operative weight loss (8). As such, 2–6 weeks of intense pre-operative dieting is worth considering in the higher risk patients. In our study, all our patients had normal liver function prior and post- surgery. Patients were also kept on a low-calorie diet with Optifast for 2 weeks prior to undergoing surgery, weight loss was encouraged although this was not mandated.
The most common cause of bleeding following LSG is SLB (9). Consensus are that the surgical staples, including technique used, play a crucial role in minimizing the risk of post-operative bleeds. With increased stapling device available in the market, a surgeon must be thoroughly familiar with the device to ensure successful outcomes.
It is generally not appreciated that the thickness of the gastric wall differs in various parts of the stomach. It is usually thickest at the antrum and becomes progressively thinner towards the fundus (10). In addition, this thickness is confounded by factors such as sex and race. Hence, a single staple height cannot be used to effectively oppose the entire length gastric wall thicknesses. An expert knowledge of staple heights from the respective equipment manufacturers is required and the selection of the appropriate staple height must be balanced between the risk of leak versus bleeding. Too short a staple height will result in opening of the edges immediately or later if ischemia occurs. It is generally safer to err on a larger stapler height as preventing a leak takes precedence over bleeding. Since bleeding over the staple line can be managed with suture or clip haemostasis.
To mitigate the risks of staple line leak and bleeding, staple line reinforcement has been advocated to increase staple line tension force as well as providing haemostasis (9). Several intra-operative methods have been adopted (11) such as buttressing, which works by employing a buttressing material that strengthens and provides a compressive effect on the staple line and improving haemostasis. Other methods include reinforcing with sutures with or without omentopexy, fibrin spray or roofing the staple line with gelatin matrix. A definitive joint decision, however, has not been reached (12).
Divided sentiments of the best method persist. In a prospective randomized trial, Dapri and colleagues (13) rivalled the incidence of SLB from LSG using 3 different techniques: stapling the stomach with no reinforcement, reinforcement with suturing and reinforcing with buttressing. These revealed a significantly lower rate of bleeding with the use of buttressing material. A separate trial by Albanopoulos and colleagues (14), conversely, did not identify a substantial distinction in the frequency of post-operative bleeding between patients where buttressing the staple line at the gastroesophageal junction (angle of Hiss) and patients who received staple-line suturing. Ultimately, because there is no clear benefit for staple line reinforcement, the additional cost of implementing such methods must be weighed against the potential benefit in bleeding reduction (12).
Post-operative bleeding can manifest as an intra- or extra-luminal bleed. Interventions required to achieve haemostasis depends on the source of this bleeding.
Intraluminal bleeding may present as an upper gastrointestinal bleed with symptoms such as hematemesis or melena. Diagnosis and management of intraluminal bleeding follows the common algorithm taken for an upper gastrointestinal bleed. This includes establishment of large bore intravenous lines for fluid resuscitation, administration of packed red blood cells if necessary, accurate measurement of urine output with insertion of a Foley catheter and urgent endoscopic intervention in the form of gastroscopy to diagnose and control the source of bleeding (3), usually by a combination of adrenaline injection and clips.
Conversely, common sources for extraluminal bleeding involve the gastric staple line, spleen, liver or abdominal wall at the sites of trocar entry. This can present insidiously with a sequential drop in serum haemoglobin levels or signs of tachycardia or hypotension and entails a high degree of suspicion for diagnosis. A second-look laparoscopy for patients presenting with extraluminal bleeding with a sustained heart rate greater than 120 beats per minute and fall in haemoglobin of more than 10 g/L post-operatively is recommended. This is because urgent laparoscopy can accelerate a diagnosis and function as a therapeutic intervention. Therapeutic intervention includes evacuation of the clot and achieving surgical control of the source of bleeding This is shown by the 2 patients in our study that had their post-operative bleed successfully managed through surgical means.
However, a second-look laparoscopy is not without its limitations. This is because the actual source of bleed may be difficult to be identified, due to increased visceral fat limiting visualisation, or intermittent bleeding. In such cases, re-operating on the patient might prove to be futile in achieving haemostasis.
Nevertheless, should re-operation be performed, and the bleed cannot be localized, other therapeutic steps can be taken. In these patients, evacuation of the hematoma and placement of a closed suction drain can help monitor for further bleeding. The patient can subsequently be imaged to localise the source of the bleed.
Other than operative interventions, imaging via computed tomography (CT) angiogram maybe considered an alternative first line modality in a patient presenting with a post-gastrectomy bleed, provided the patient is stable. This is because CT angiogram will permit localisation of the source of bleed and allow for targeted intervention. This might prove more rewarding in patients with a lot of visceral fat that make localizing bleeding source difficult. This is seen in one of our patients, where operative means proved futile but CT angiography performed post-operatively successfully picked up the active contrast bleeding. However, this decision is not easy and clinical judgement must be made considering the stability of patient and available resources.
Notably, apart from being able to visualize the bleed, this management route offers the opportunity for endovascular interventions (15). However, interventional radiology expertise must be available and is akin to managing acute abdominal or gastrointestinal bleeding. Should the bleeding be due to SLB, endovascular haemostasis is unlikely to be successful and surgical haemostasis is then indicated.
As seen in this study, 2 patients were treated with non-operative means. Conservative treatment in such situations is achieved through fluid resuscitating the patient and blood transfusion. As seen in Sroka et al.’s observational report (1), the majority of post-operative bleeds were treated conservatively. However, this is only considered in patients who are stable (16). This requires careful monitoring of the patient’s vitals, and is also dependent on the level of comfort of the surgeon.
Other means of decreasing post-operative bleeding in LSG include elevating blood pressure and removing bougie (may cause compression) when inspecting staple line for haemostasis, and the use of tranexamic acid (1,5).
In summary, LSG is an effective metabolic and bariatric procedure rapidly gaining in popularity. Therefore, the number of patients undergoing this procedure will continue to rise. Basic understanding of bleeding complications and available treatment options is essential for all practising surgeons. Management of post-operative bleeding requires clinical judgement. Bleeding may stop spontaneously or require haemostasis usually by operative means. In cases where localization of the source of bleed is difficult, CT angiogram to identify source of bleeding and subsequent embolization is an option.
Conclusions
Post-operative bleeding after LSG occurred at the staple line and branches of the right gastro-epiploic artery and short gastric arteries. Management of post-operative bleeding requires clinical judgement. Bleeding may stop spontaneously or require haemostasis by operative or endovascular means. CT angiogram is proposed to locate the source of bleed and subsequent embolization of bleeding arteries maybe possible, avoiding the need for re-operation. However, bleeding from the staple line requires surgical haemostasis.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr.2019.04.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sroka G, Milevski D, Shteinberg D, et al. Minimizing Hemorrhagic Complications in Laparoscopic Sleeve Gastrectomy--a Randomized Controlled Trial. Obes Surg 2015;25:1577-83. [Crossref] [PubMed]
- Catheline J, Fysekidis M, Dbouk R, et al. Weight Loss after Sleeve Gastrectomy in Super Superobesity. J Obes 2012;2012.
- Sarkhosh K, Birch DW, Sharma A, et al. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon’s guide. Can J Surg 2013;56:347-52. [Crossref] [PubMed]
- Jossart GH. Complications of Sleeve Gastrectomy: Bleeding and Prevention. Surg Laparosc Endosc Percutan Tech 2010;20:146-7. [Crossref] [PubMed]
- Chakravartty S, Sarma DR, Chang A, et al. Staple Line Bleeding in Sleeve Gastrectomy-a Simple and Cost-Effective Solution. Obes Surg 2016;26:1422-8. [Crossref] [PubMed]
- Deitel M, Crosby RD, Gagner M. The First International Consensus Summit for Sleeve Gastrectomy (SG), New York City, October 25-27, 2007. Obes Surg 2008;18:487-96. [Crossref] [PubMed]
- Fris RJ. Preoperative low energy diet diminishes liver size. Obes Surg 2004;14:1165-70. [Crossref] [PubMed]
- Edholm D, Kullberg J, Haenni A, et al. Preoperative 4-week low-calorie diet reduces liver volume and intrahepatic fat, and facilitates laparoscopic gastric bypass in morbidly obese. Obes Surg 2011;21:345-50. [Crossref] [PubMed]
- Janik MR, Walędziak M, Brągoszewski J, et al. Prediction Model for Hemorrhagic Complications after Laparoscopic Sleeve Gastrectomy: Development of SLEEVE BLEED Calculator. Obes Surg 2017;27:968-72. [Crossref] [PubMed]
- Huang R, Gagner M. A Thickness Calibration Device Is Needed to Determine Staple Height and Avoid Leaks in Laparoscopic Sleeve Gastrectomy. Obes Surg 2015;25:2360-7. [Crossref] [PubMed]
- Musella M, Milone M, Maietta P, et al. Laparoscopic sleeve gastrectomy: efficacy of fibrin sealant in reducing postoperative bleeding. A randomized controlled trial. Updates in Surgery 2014;66:197-201. [Crossref] [PubMed]
- Bülbüller N, Aslaner A, Öner OZ, et al. Comparison of four different methods in staple line reinforcement during laparascopic sleeve gastrectomy. Int J Clin Exp Med 2013;6:985-90. [PubMed]
- Dapri G, Cadiere GB, Himpens J. Reinforcing the staple line during laparoscopic sleeve gastrectomy: prospective randomized clinical study comparing three different techniques. Obes Surg 2010;20:462-7. [Crossref] [PubMed]
- Albanopoulos K, Alevizos L, Flessas J, et al. Reinforcing the staple line during laparoscopic sleeve gastrectomy: prospective randomized clinical study comparing two different techniques. Preliminary results. Obes Surg 2012;22:42-6. [Crossref] [PubMed]
- Vaidya S, Tozer KR, Chen J. An Overview of Embolic Agents. Semin Intervent Radiol 2008;25:204-15. [Crossref] [PubMed]
- Hussain A. EL-Hasani S. Bariatric emergencies: current evidence and strategies of management. World J Emerg Surg 2013;8:58. [Crossref] [PubMed]
Cite this article as: Yong S, Poh B, Eng A, Pasupathy S, Chan WH, Lee PC, Too CW, Urlings T, Tham KW, Lim EKW. Post-operative bleeding complications in laparoscopic sleeve gastrectomy: sources, solutions and lessons learnt from a single cohort of patients. Dig Med Res 2019;2:6.